Richmond, Va. – The OPTN Board of Directors, at its meeting June 10, approved new geographic areas to match heart and vascularized composite allograft (VCA) transplant candidates with available organs from deceased donors. Both systems will now be based on distance from the donor hospital to the transplant hospital, replacing fixed and irregular donation service area (DSA) and regional boundaries.
“We continue to refine the organ distribution process to create the most equitable access for all people in need of a transplant,” said Sue Dunn, president of the OPTN Board. “The new distribution systems will help us do so, and they have been well supported by clinicians and the public. They also are in keeping with federal regulatory guidance to minimize differences in transplant access based on where candidates live or which transplant hospital they choose.”
Under the revised policy for heart and lung allocation, the most local level of heart distribution will be for transplant candidates listed at hospitals located within 250 nautical miles of the donor hospital. This replaces all references to the hospitals within the DSA where the heart is recovered. Lung distribution policy was updated in November 2017 to replace local DSA boundaries with the same distribution area (transplant candidates listed at programs within 250 nautical miles of the donor hospital).
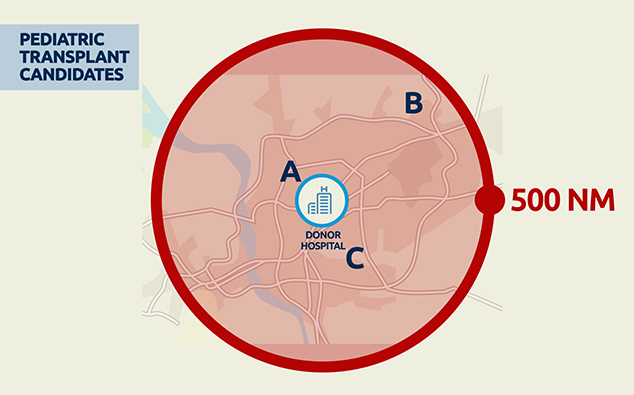
VCA distribution will now begin with compatible candidates listed at transplant hospitals within a 500 nautical mile distance from the donor hospital. The great majority of VCA transplants performed up until now have come from donors within this distance.
Other key actions
In other action, the OPTN Board approved an amendment to the OPTN’s variance for the HIV Organ Policy Equity (HOPE) Act. Programs meeting HOPE Act research and experience requirements will now be able to recover additional organ types from donors identified as HIV-positive and transplant them into candidates who are also HIV-positive. This measure broadens organs transplantable from liver and kidney to also include heart, lung, pancreas, kidney-pancreas and intestinal organs.
The Board amended a proposed voluntary national protocol encouraging the greater use of split liver transplants (dividing a liver from a deceased donor into two segments that may be transplanted into two different candidates). After discussion, the Board approved the protocol for use in OPTN Region 8, which had requested it on a regional basis prior to the proposed national variance. Other regions may apply later for a similar protocol if they choose.
The Board also endorsed two guidance documents: one outlining ethical implications of multi-organ transplants and one describing effective practices in broader organ distribution.
About the OPTN Board of Directors
The Organ Procurement and Transplantation Network (OPTN) Board of Directors establishes and updates OPTN policies and bylaws, consistent with statutory and regulatory requirements. The Board consists of 42 volunteer experts from around the country in organ procurement and transplantation as well as organ recipients, living donors, and donor families. The Board is supported by the efforts and recommendations of 21 OPTN committees, also comprised of volunteers representing the diversity of people, perspectives and expertise involved in the nation’s organ transplant network.
United Network for Organ Sharing (UNOS) serves as the OPTN under federal contract.
The post appeared first on UNOS.




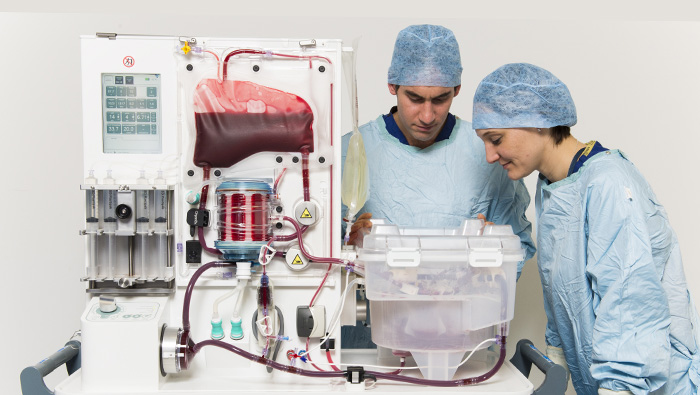


 ) Transportable NMP
) Transportable NMP