Industry Literature
Preservation for heart transplantation is actively evolving. Stay abreast of news with this helpful resource for informative publications. The following industry specific literature citations are selected for the content of donor heart preservation and perfusion. The purpose of this curated information is to provide a trusted guide to emerging high-value information from a dedicated and sustained critical search of thousands of news sources.

INSIGHTS
UNOS Chief Medical Officer David Klassen, M.D., discusses perfusion-driven advances and remaining challenges
Q. Where is perfusion having the most impact right now in organ transplantation?
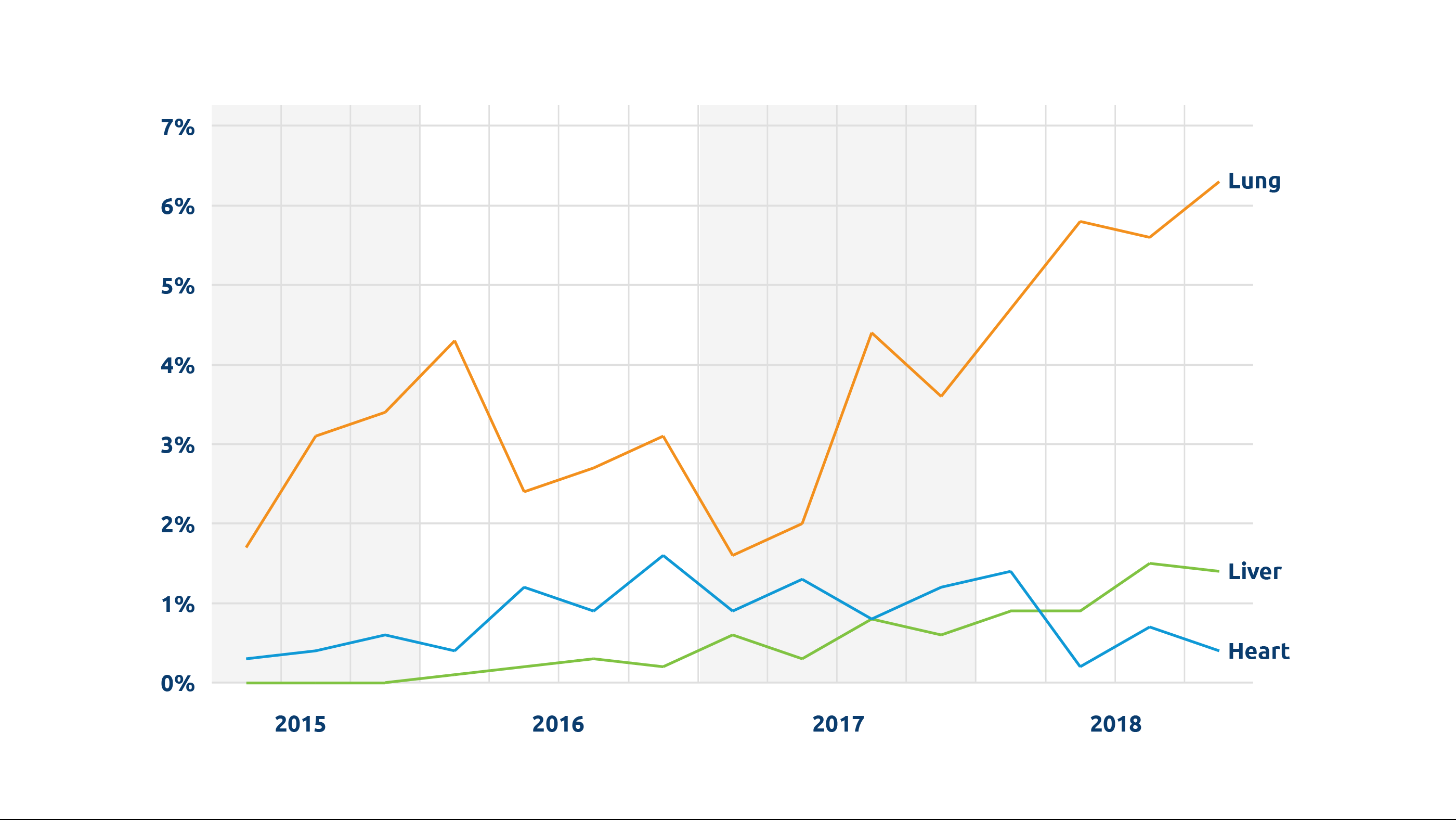
We’re seeing a steady increase in the number of organs being perfused. In 2022, more than 1,100 transplanted donor livers, hearts and lungs were perfused. Between 2018 and 2022, there was an almost five-fold increase in perfused livers transplanted, from fewer than 100 to nearly 500.
Perhaps the most important recent development is perfusion for DCD (donation after circulatory death) heart transplantation. Because the heart is particularly vulnerable to warm ischemic injury, increasing the number of successful DCD heart transplants was really dependent on perfusion technologies. A third of all donors are DCD donors, and potentially a significant number could be heart donors; one study estimated that widespread adoption of DCD heart transplant could lead to 300 more adult heart transplants annually. UNOS data show that the number of DCD hearts perfused and transplanted has gone from 0 in 2018 to 199 in 2022.

“Perhaps the most important recent development is perfusion for DCD (donation after circulatory death) heart transplantation.”
David Klassen, M.D., Chief Medical Officer
Q. Are there other significant new developments?
In addition to ex-vivo machine perfusion, normothermic regional perfusion (NRP) for recovery of organs from DCD donors is now spreading fairly widely, and is also helping to increase the number of DCD hearts available for transplant. (See Normothermic Regional Perfusion: A reading list.) With NRP, cardiopulmonary bypass or ECMO is used to restore circulation and enable perfusion of DCD organs prior to recovery. Because these technologies are already in use in most ICUs, there is no new technology to acquire or learn to use, as there is with ex-vivo machines. Also with NRP, separate perfusion devices for each organ aren’t needed, as abdominal and thoracic organs can be perfused before recovery with this process.
Q. What barriers remain to greater use of perfusion?
At the present time cost remains the most significant limiting factor for either type of perfusion. It’s expensive, and questions have yet to be resolved about when it is most appropriate to use perfusion, who pays for it, and how those costs are reimbursed. Whether cost will limit the use of perfusion to larger and better-resourced transplant centers or OPOs remains to be seen.
Implementation of these technologies is also logistically complex. It takes time and effort for new technologies and procedures like these to be widely incorporated.
Finally, more data are needed to firmly determine whether perfusion leads to better patient outcomes. Research so far indicates generally equal outcomes when compared with non-perfused organs. Despite these questions perfusion is enabling increased use of expanded criteria and DCD organs, including those that previously might not have been considered viable for transplant.
A reading list
Understanding Normothermic Regional Perfusion (NRP)
Rather than perfusing donor organs by machine after recovery (“ex-vivo” perfusion), NRP uses extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass technology to restore circulation and perfuse DCD donor organs prior to recovery from the deceased donor. The advantages of NRP include the potential to reduce warm ischemia time for DCD donor organs and the ability to assess DCD hearts prior to recovery.
However, NRP is technically complex and requires rapid, coordinated execution by a skilled team. To ensure success with the procedure, the recovery team may need to bring all the necessary equipment and supplies, as well as its own perfusionists, which can add to the cost and other considerations of procurement.
In addition, questions have been raised even within the medical community about the ethics of a procedure that restores circulation in a deceased donor as well as about the transparency necessary for true informed consent from donor families.
This reading list provides an overview of NRP as well as discussions and recent perfusion news coverage.
The Organ Donation and Transplantation Alliance: “Introduction to NRP and Perfusion in DCD: What Do These Concepts Mean?”
American Society of Transplantation Position Statement on Normothermic Regional Perfusion
Nov. 2021 | The Journal of Heart and Lung Transplantation: Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion
June 2022 | Cureus: Normothermic Regional Perfusion is an Emerging Cost-Effective Alternative in Donation After Circulatory Death (DCD) in Heart Transplantation
April 2022 | The Journal of Heart and Lung Transplantation: Hospital Administration Considerations for Implementation of Normothermic Regional Perfusion DCD Heart Transplant Program
March 2022 | The Organ Donation and Transplantation Alliance: “DCD in Heart Transplant – The Case for Normothermic Regional Perfusion” (video)
Oct. 2022 | American Society of Anesthesiologists: “Statement on Controlled Organ Donation After Circulatory Death”
April 2021 | The American College of Physicians: “The American College of Physicians says organ procurement method raises significant ethical concerns”
Feb. 2020 | The Journal of Medicine and Philosophy: A Forum for Bioethics and Philosophy of Medicine: “Why DCD donors are dead” An ethical and philosophical analysis of NRP and DCD transplant.
Nov. 2022 | Mayo Clinic News Release: “Beating the Odds for a transplant”
Sept. 2022 | In tctMD from the Cardiovascular Research Foundation: “Heart Transplantation After Circulatory Death Gives ‘Encouraging’ Results”
Sept. 2022 | UC San Diego Health press release: “Father’s Life is Saved after Receiving Heart, Kidney and Liver Transplant”
May 2022 | KFOR news: “Oklahoma organ team makes history with first successful perfusion liver transplant”
Dec. 2021 | “Medical City Heart Hospital performs first DCD transplant in TX”
Jan. 2020 | PR Newswire: “NYU Langone Performs First U.S. Heart Transplant Using Novel Organ Revitalization Technique”
Dec. 2019 | CNN: “Doctors ‘reanimate’ heart for first-of-its-kind transplant in US”
The post appeared first on UNOS.
in focus
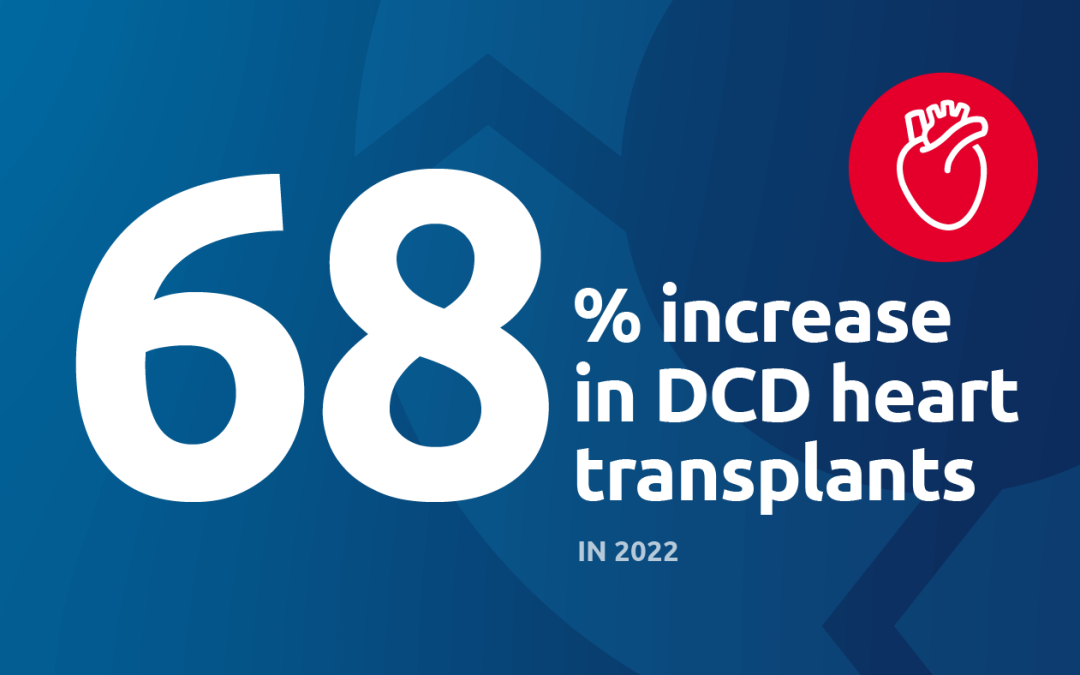
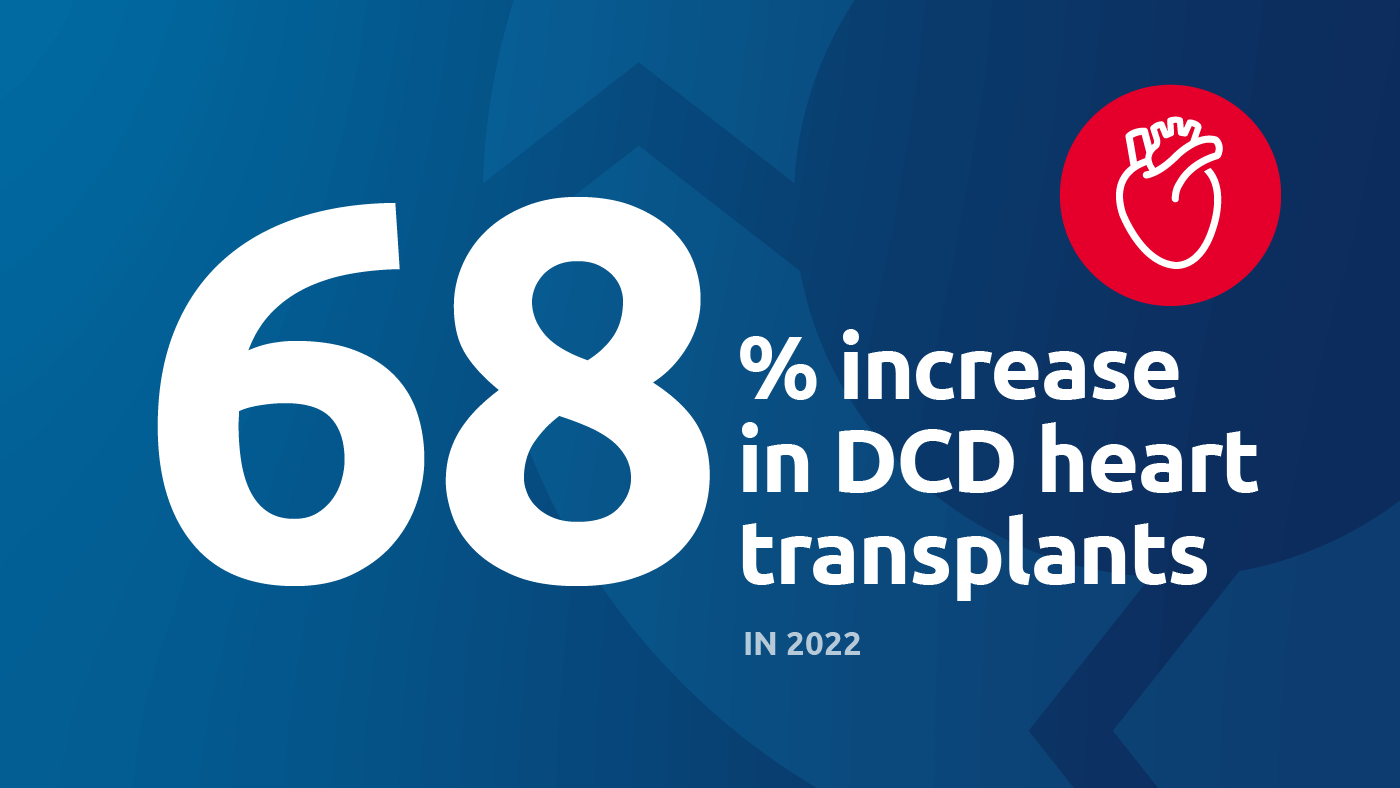
A 68% increase in DCD heart transplants was part of a record-setting 2022
The 11th straight year of increases in heart transplants coincides with advancements in organ perfusion technology and DCD recovery practices.
In 2022, 42,888 organ transplants were performed in the United States, an increase of 3.7 percent over 2021 and a new annual overall record.*
While new records were also set for liver, kidney and lung transplants, heart transplants in particular experienced a steep increase, from both donation after brain death (DBD) donors, as well as donation after circulatory death (DCD) donors.
Heart transplants increased overall by 21.5 percent (4,169 in 2022)
DBD heart transplants increased 4.6 percent (3,822 in 2022)
DCD heart transplants increased 68 percent (347 in 2022)
Advances in technology and donor recovery practices contributing to increases
Rapidly-evolving perfusion technology is allowing more DCD hearts to be transplanted. Perfusion allows organs to remain viable for longer periods outside the body; this is important for organs such as hearts and lungs, which have shorter windows of time when compared to kidneys. 2022 saw a 95 percent increase in transplants of machine-perfused hearts.
Coinciding with these advances in technology, increasing recovery of DCD donors has been a key area of focus for the nation’s 56 organ procurement organizations (OPOs) for a number of years. A recent UNOS-led collaborative project helped OPOs share effective practices related to recovering DCD donors to increase transplant. Over the course of the national project, 75 percent of OPOs participated in one or both of the two cohorts, contributing to the overall increases in DCD donors recovered and DCD organs transplanted. A subsequent collaborative project is currently focused on increasing transplantation of DCD lungs, and more than 40 percent of the nation’s lung transplant programs are participating.
A report from the National Academies of Sciences, Engineering and Medicine (NASEM) recommends taking collaborative improvement approaches as well as embracing innovative technologies to maximize organ use, in particular use of DCD organs.
February is American Heart Month. Get resources, fact sheets and other information on the National Institutes of Health website.
*According to the most recent data from the Organ Procurement and Transplantation Network (accessed Feb. 13, 2023)
In focus
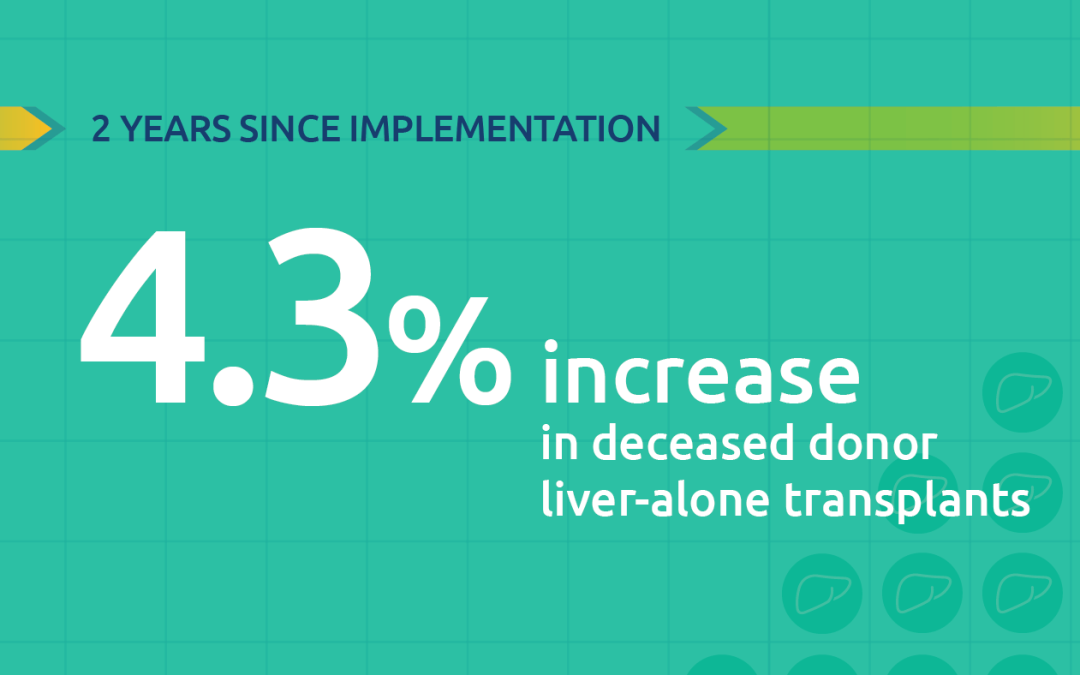
More than 15,000 liver transplants performed in first two years of acuity circles policy
Two-year monitoring shows many states had volume changes within 10 percent of previous policy.
7,000 organs tracked with UNOS Organ Tracking Service
30% of all OPOs use the UNOS Organ Tracking Service to monitor organs in transit.

7 years of HOPE
Implemented in 2015 , the HIV Organ Policy Equity (HOPE) Act has given more than 350 living with HIV an opportunity to receive a lifesaving transplant from an HIV-positive donor.
The post appeared first on UNOS.
United Network for Organ Sharing (UNOS) today announced that CEO Brian Shepard will depart the organization at the end of September, following the completion of his contract. Shepard’s 10-year tenure as UNOS CEO was marked by groundbreaking progress in the U.S. organ donation and transplantation system.
Maureen McBride, Ph.D., UNOS’ chief operating officer, will assume the role of interim CEO beginning Oct. 1 while UNOS conducts a national search for Shepard’s successor. McBride has been with the organization since 1995. She served as director of research until 2014, when she accepted her current role as COO.

A commitment to improving the system
During his tenure, Shepard presided over the adoption of innovative policies, lifesaving improvements and record increases in both organ donation and transplant, including 2021, when the national system conducted more than 41,000 transplants in a single year, a global record. These and other advancements have positioned UNOS to drive the next phase of system progress, from increasing equity in transplant to adopting cutting-edge technologies, to collaborative improvement, further strengthening the nation’s high performing system and saving more lives.
“As UNOS CEO, Brian was a constant and courageous advocate for increasing equity in our national donation and transplantation system,” said Jerry McCauley, M.D., vice-president of the UNOS Board of Directors and incoming president. “His leadership has resulted in marked improvements in access to transplant for patients of color and those who have been historically marginalized. I am proud to have worked alongside Brian as a member of the UNOS board and am excited to build upon the foundation he has laid to further advance our mission and save even more lives.”
“UNOS is the engine that powers the U.S. donation and transplant system, and we are so lucky to have had Brian Shepard in the driver’s seat for the past decade,” said Matthew Cooper, M.D., president of the UNOS Board of Directors. “During such a pivotal time in our community, Brian took UNOS to the next level, driving accomplishments and championing the work of so many. His is a legacy to be celebrated.”
Prioritizing patients, equity and innovation
Under Shepard’s leadership, UNOS undertook a series of efforts to increase equitable access to transplant, including adopting a new way to distribute donor organs that emphasizes patient need. These new polices have resulted in greater access for the sickest patients.
“These changes to organ distribution weren’t easy or always popular, and it was so important to have Brian centering these discussions,” said David Mulligan, M.D., immediate past president of the UNOS board. “Now that these policies are in place, we can see the positive impact they’re having on patients and families across the country.”
Additionally, Shepard was instrumental in the development of UNOS Labs, an innovation center dedicated to fostering new ideas and encouraging experimentation. Since its founding, UNOS Labs has developed transplant-focused predictive analytics to help doctors decide whether to accept an organ offer for their patient, a GPS tracker for organ shipments, an offer simulator to conduct behavioral science research to improve organ matching, and a high-quality medical image sharing platform.
“The UNOS team is the most incredibly talented and dedicated team I’ve ever had the honor of being a part of,” said Shepard. “I’ve always viewed my job as making their job easier; removing obstacles and watching them run. I’m so proud of what they’ve accomplished and of all of the ongoing efforts that will further improve donation and transplant in the U.S.”
A vision for the future of organ allocation
Over the last several years, Shepard has helped put into place a new allocation policy, called continuous distribution. This innovative approach dissolves rigid boundaries, and is structured so that no single attribute determines whether or not a patient receives a transplant. Importantly, continuous distribution is also designed to allow for more patient engagement in the decision-making process.
“As a three-decade heart transplant survivor who strongly advocates increased involvement for transplant patients in the policy development process, continuous distribution is a game changer,” said Jim Gleason, president of Transplant Recipients International Organization (TRIO). Gleason has engaged with UNOS for more than 25 years and is a two-term former UNOS Board member. “This effort is not only going to help guide patients to the information they need in their transplant journey, it will also give them an active contributor seat at the decision-making table.”
A lasting legacy
“From policymaking to technology, from system-wide improvements to one-on-one interactions, Brian’s leadership has left an indelible mark on UNOS and the wider donation and transplant community,” said Sue Dunn, former CEO of Donor Alliance and a former UNOS board president. “But for me, to see his ongoing commitment to honoring selfless donors, their courageous families, and recognizing the often-thankless work of our OPOs – that is a legacy be proud of.”
“We’ve come such a long way in the last decade,” said Shepard. “While I am honored that the Board asked me to continue to serve as CEO, I felt it was the right time to take the next step. I have worked with so many amazing and dedicated people over the years who made it possible to accomplish all that I originally set out to do as UNOS CEO. Now, as we embark on a new chapter with even more exciting opportunities, I know the UNOS team and the donation and transplant community are in good hands, and I’m excited about the future.”
The post appeared first on UNOS.
Transplant Direct. 2021 Feb 22;7(3):e676. doi: 10.1097/TXD.0000000000001120. eCollection 2021 Mar.
ABSTRACT
Organ donation after euthanasia is performed in an increasing number of countries. In this donation after circulatory death procedure, it has not been possible to donate the heart. Recent literature, however, reports positive results of heart donation after circulatory death. Therefore, patients who donate organs following euthanasia might be suitable candidates for heart donation. We want to confirm this assumption by sharing the results of 2 cases of heart donation following euthanasia with ex situ subnormothermic heart preservation. Our aim is to raise awareness of the potential of heart donation following euthanasia for both clinical transplantation and research.
METHODS: The data of 2 consecutive heart donations following euthanasia were collected prospectively. Informed consent was obtained from the patients themselves for heart donation for research purposes. An acellular oxygenated subnormothermic machine perfusion strategy was used to preserve both donor hearts. Subsequently, the hearts were evaluated on a normothermic perfusion machine using a balloon in the left ventricle.
RESULTS: Heart donation following euthanasia was feasible without significant changes in existing retrieval protocols. Duration of machine perfusion preservation was 408 and 432 minutes, for heart 1 and 2, respectively. For heart 1, developed pressure (Pdev) was 119 mm Hg, maximal rate of pressure rise (dP/dtmax), and fall (dP/dtmin) were 1524 mm Hg/s and -1057 mm Hg/s, respectively. For heart 2, Pdev was 142 mm Hg, dP/dtmax was 1098 mm Hg/s, and dP/dtmin was -802 mm Hg/s.
CONCLUSIONS: Hearts donated following euthanasia are highly valuable for research purposes and can have sufficient quality to be transplanted. With the implementation of ex situ heart perfusion, patients who are to donate their organs following euthanasia should also be able to donate their hearts. The complex combination of euthanasia and heart donation is ethically sound and surgically feasible and can contribute to shortening the heart transplant waiting list.
PMID:34104712 | PMC:PMC8183709 | DOI:10.1097/TXD.0000000000001120
J Card Surg. 2021 Jul;36(7):2596-2597. doi: 10.1111/jocs.15520. Epub 2021 Mar 30.
ABSTRACT
Throughout the world, a shortage of donor organs has prompted development of unique strategies to expand the donor pool. Here, we review a report by Medressova and colleagues to the Journal of Cardiac Surgery detailing the 3-year follow-up of a patient who successfully underwent a heart transplant after 17 hours of ex-vivo preservation.
PMID:33783039 | PMC:PMC8187278 | DOI:10.1111/jocs.15520
The winter 2021 public comment cycle opens Jan. 21 and will close March 23. The Organ Procurement and Transplantation Network (OPTN) is offering six proposals, two requests for feedback, and one white paper for public comment.
Comments and replies will be published here on the OPTN website to promote transparency and trust in the national transplant system. Visitors also have the option to share their posted comments to social media.
We encourage patients, transplant candidates and recipients, living donors, donor families and transplant professionals to learn more about the proposals and provide their valuable feedback to help shape U.S. organ transplant policy.
Items available for public comment:
- 2021-2024 OPTN Strategic Plan
- Calculate Median MELD at Transplant around the Donor Hospital and Update Sorting within Liver Allocation
- Clarify Multi-Organ Allocation Policy
- Develop Measures for Primary Graft Dysfunction in Hearts
- Modify the Deceased Donor Registration (DDR) Form
- Require Notification of Human Leukocyte Antigen (HLA) Typing Changes
- Revise General Considerations in Assessment for Transplant Candidacy
- Update National Liver Review Board Guidance Documents and Policy Clarification
- Update Transplant Program Key Personnel Training and Experience Requirements
Educational resources will be made available January 21 to provide multiple opportunities to learn more about the proposals.
All comments are reviewed and considered by the OPTN Board of Directors before they vote on the proposals to become policy. Learn more about the policy development process here.
The post appeared first on UNOS.
This holiday season, organ placement specialists are working around the clock in the UNOS Organ Center to help place lifesaving organs across the country. Here’s their holiday message for everyone touched by transplant. We wish you all a healthy and peaceful New Year!
The post appeared first on UNOS.
Pediatric transplant program components
The Board of Directors of the Organ Procurement and Transplantation Network, at a virtual meeting held Dec. 7, approved pediatric components for 268 heart, kidney, liver, lung and pancreas transplant programs. Effective Dec. 8, any candidate younger than age 18 must be listed at one of these programs unless an exception is made for a very medically urgent heart or liver candidate. The searchable member directory on the OPTN website will display programs with a pediatric component.
“This is a key milestone in promoting the safety and efficiency of transplantation for children in need of a transplant,” said David Mulligan, M.D., president of the board. “The requirements were developed carefully to ensure that these programs have highly trained and experienced clinical staff and appropriate facilities to care for the specific needs of pediatric candidates and recipients. The application and review process took place in a staged fashion to allow programs interested in applying to take any needed steps to ensure they would qualify.”
Strategic planning
The board heard an overview of ongoing development of the OPTN Strategic Plan for 2021 through 2024. The OPTN Executive Committee will circulate a draft plan for public comment in January 2021, and a proposed final plan will be presented for board action in June 2021. As currently envisioned, there are four overall strategic goals:
- Increase the number of transplants
- Provide equity in access to transplants
- Promote living donor and transplant recipient safety
- Improve waitlisted patient, living donor, and transplant recipient outcomes
COVID-19 operational actions reviewed, will remain in effect
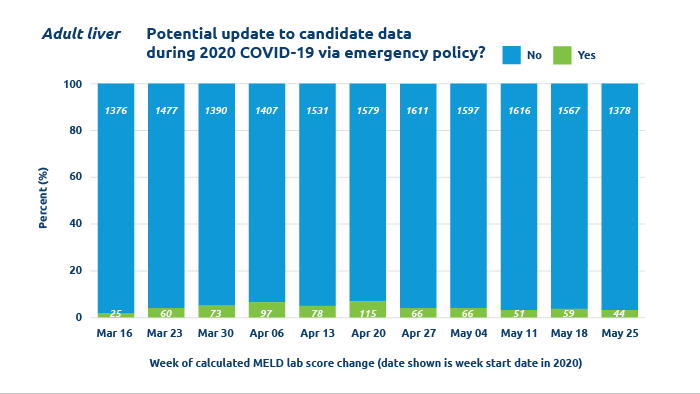
The board reviewed several operational actions adopted by the OPTN Executive Committee in March and April, 2020, to help members document COVID 19-issues affecting organ donation and transplantation and to help members focus needed resources on essential clinical services. The board agreed to make permanent a requirement for OPTN members to document COVID-19 testing for all potential deceased donors. The board resolved that the following measures will remain in effect, subject to ongoing Executive Committee review for their applicability and effectiveness:
- Updates to transplant candidate data if a transplant hospital is unable to bring a candidate in for updated lab testing due to COVID-19 issues
- Relaxation of certain data submission requirements for follow-up of transplant recipients and living donors
- Modifications to reporting wait time initiation for kidney transplant candidates who are not on dialysis
Other actions
The board took additional actions as follows:
- Approved new and amended OPTN policies to align with recently updated recommendations from the U.S. Public Health Service to assess organ donors and monitor transplant recipients for potential HIV, hepatitis B and hepatitis C infection
- Endorsed a slate of nominees for election to open positions on the board for terms beginning July 1, 2021
- Approved the programming into the UNetSM system of allocation policy for vascularized composite allografts (VCA)
- Modified data collection requirements for living VCA donors
- Accepted new guidance and updated policy regarding adult heart allocation, to standardize and streamline data reporting for candidates with certain clinical conditions
- Adopted guidance addressing the use of exception requests for pediatric heart candidates
- Approved additional updates to operational processes and guidance for the National Liver Review Board, to clarify guidance and better ensure expert review of exception requests
- Updated the cohort of data used to calculate the lung allocation score
- Amended the OPTN Bylaws to permit all members of the board to vote on the full slate of representatives to the OPTN Executive Committee
The post appeared first on UNOS.
Implementation date:
- Dec. 3: Heart, lung, heart-lung, liver and intestine
At-a-glance
For heart, lung, heart-lung, liver and intestine programs, local donor acceptance criteria in Waitlist℠ have been updated in order to provide additional efficiency in organ allocation. Transplant programs should evaluate their current settings.
Summary of changes
With the transition away from donation service area (DSA) as a unit of allocation, a new framework has been developed to determine what type of offers would be screened using “local” acceptance criteria for a candidate. Because proximity to the donor hospital is a primary factor in the revised allocations, changes have been made to allow the location of the donor hospital in relation to the candidate to be considered when “local” acceptance criteria are applied. Read additional background information here.
For each organ type, the following new local acceptance criteria will be used to include candidates on the match run:
- Offers to candidates listed at transplant programs within the DSA and/or within 250 NM of the donor hospital:
- Heart
- Heart-lung
- Lung
- Offers to candidates listed at transplant programs within the DSA and/or within 150 NM of the donor hospital:
- Liver
- Offers to candidates listed at transplant programs within the DSA and/or within 500 NM of the donor hospital:
- Intestine
Similar changes will be implemented for kidney and pancreas allocation on Dec. 15, when DSA and region are removed from distribution of those organs.
The inclusion of DSA in this definition does not impact the order of the match run as that is established and organized by distance-based allocation definitions within OPTN policy.
What you need to do
Transplant programs for all organ types should evaluate their current “local” acceptance criteria settings for candidates in Waitlist and determine if updates are appropriate based on the revised definition of “local” donor acceptance criteria.
Additional resources
Find professional education on UNOS Connect:
- QLT103D Acceptance Criteria for Distance-based Allocation
In addition, DonorNet® online help documentation has been updated so that transplant programs will have access to the information about how local acceptance criteria is used for offers for all organ types.
Questions?
If you have questions relating to implementation, contact UNOS Customer Service at unethelpdesk@unos.org, or call 1-800-978-4334 from 8 a.m. to 7 p.m. EST.
The post appeared first on UNOS.
This final rule revises the Organ Procurement Organizations (OPOs) Conditions for Coverage (CfCs) to increase donation rates and organ transplantation rates by replacing the current outcome measures with new transparent, reliable, and objective outcome measures and increasing competition for open donation service areas (DSAs).

Innovation: FEATURE
UNOS Labs is a collaborative space where scientists, researchers and technology experts partner with donation and transplantation professionals to develop new solutions aimed at increasing efficiency in the national transplant system and saving more lives.
UNOS LabsSM , an experimental incubator created in 2018, fills a critical role in bridging resources and research expertise to make innovation happen and bring big ideas to life. It enables our researchers to develop solutions to continuously improve the national organ donation and transplant system and increase organ utilization.
“We’ve begun this endeavor of UNOS Labs to address some of the bigger questions in transplant and try to meet the community where they are, and try and capitalize on a lot of the innovations that are out in the medical space,” said program manager Casey Humphries.
UNOS Labs explores and validates concepts and technologies that may be deployed across the nation’s organ transplant system. It is a place to try out new ideas under the primary pillars of behavioral research, data science and technology through extensive testing and incremental development.
“The emphasis in Labs is that projects have an end goal,” Humphries said. “They’re not academic, they’re not something that we want to just publish on and then store under the pillow. The goal of Labs is to create something that can be used to benefit the system.”
Improving the transplant system through innovation
Among the new technologies UNOS researchers are leveraging is natural language processing (NLP) to predict which deceased donor kidneys will experience placement difficulties and to forecast which will be accepted by transplant hospitals. Encouraged by the study findings, the UNOS team plans to conduct more research to better understand the potential to enhance existing predictive models.
Humphries said a unique process such as NLP is a great example of data science.
“We have data science, and that’s really looking at understanding data broadly and looking at innovative ways to approach big data,” she said. “We can derive insights from using data science techniques…something that we can’t do with typical statistics.”
UNOS researchers are also partnering with organ procurement organizations (OPOs) nationwide to better predict organ travel time. One of the biggest contributors to prolonged cold ischemic time is coordinating logistics for organ transport. Using data gathered from couriers and pilot OPOs, researchers are also studying the feasibility of real-time tracking of organs shipped via ground and air.
Other partnership projects involving UNOS researchers include developing a series of simulation models to study the impact of possible changes to the transplant ecosystem; measuring physicians’ attitudes and perceptions around predictive analytics; and developing a liver paired exchange pilot program.
Philanthropic support for research and analytics
The Mendez National Institute of Transplantation Foundation recently awarded UNOS a $100,000 grant toward better understanding of the role biopsies play in transplant outcomes. The research further aims to improve the tools that inform kidney offer acceptance decisions.
The F.M. Kirby Foundation has given UNOS $75,000 to help train clinicians in promoting organ donation and increase the number of organ transplants during the COVID-19 pandemic. And the Fresenius Medical Care Foundation awarded UNOS $106,000 to help improve transportation and logistics for organ donation.
“We are challenging ourselves to think more broadly about the technologies we could bring to the transplant system,” explained Humphries. “Grants make this research happen.”
The post appeared first on UNOS.
Audience
- Primary Data Coordinators, Physicians, Program Administrators and Surgeons at heart and lung programs
- Transplant Program’s Clinical Coordinators, Administrators/Managers, Program Directors, Medical Directors and Surgical Directors at heart and lung programs
- Please share this notice with anyone in your organization who would benefit from this information
Implementation date
Oct. 28, 2020
At-a-glance statement
The following changes to the diagnosis code drop down list in WaitlistSM and the Primary Diagnosis drop down list on TCR and TRR forms in TIEDI® are now available:
- Lung candidates
-
- COVID-19: ARDS
- COVID-19: PULMONARY FIBROSIS
- Heart candidates
-
- COVID-19: DILATED MYOPATHY: ACTIVE MYOCARDITIS
- COVID-19: DILATED MYOPATHY: HISTORY OF MYOCARDITIS
- DILATED MYOPATHY: VIRAL changed to DILATED MYOPATHY: VIRAL (NOT COVID-19)
- Conversion of of all actively listed Waitlist candidates with the inactive diagnosis to the new one. Removed candidates are not impacted.
- For patients with the inactivated diagnosis on TIEDI forms, their Primary Diagnosis field are blank. Members entering forms in TIEDI need to select an appropriate code for their patients with viral Dilated Myopathy from one of the three new available codes.
What you need to do
- Transplant hospitals should use the COVID-19 diagnosis codes as appropriate when listing lung and heart candidates or entering information on TCR and TRR TIEDI forms.
- Transplant hospitals should also modify the diagnosis for any patient converted to the new Dilated Myopathy: Viral (Not COVID-19) code in Waitlist who should more accurately be assigned one of the COVID-19 heart diagnosis codes.
Additional details
The purpose of adding these options is to specify when COVID-19 related organ failure is the cause for lung and heart candidate listings.
The initial proposed action, addressing lung candidate diagnoses, was published for special public comment from Aug. 31 through Oct 1. During public comment, the OPTN Heart Transplantation Committee recommended that heart candidate diagnoses be added to those originally proposed for lung candidates. The OPTN Board of Directors approved the proposal during an Oct. 8 conference call.
Having these options available helps to identify trends in these patient populations that could inform future policy changes. This is not expected to have any impact to the lung allocation score calculated for these lung candidates.
Education and resources
Read the policy notice on OPTN
Questions?
If you have questions relating to implementation, contact UNOS Customer Service at unethelpdesk@unos.org, or call 800-978-4334 from 8 a.m. to 7 p.m. EDT.
For policy-related questions, contact member.questions@unos.org.
The UNOS COVID-19 resources page is refreshed regularly with the most current updates and resources. Please check back frequently for updates.
The post appeared first on UNOS.
in focus
Registries provide analysis and information to the transplant and healthcare community, improving the quality and safety of care.
The transplant community benefits from partnering with UNOS to create registries.
Custom solutions
Contact us to learn more about Registries
The transplant community needs data warehouses that underlay new analytical tools and drive better outcomes and deeper insights. Registry-based studies make substantial contributions to the field of transplantation by optimizing the use of new data sources to understand outcomes and improve transplant management.
“UNOS’s registries enhance data collection and leverage our 35 years of transplant knowledge to analyze patient outcomes, study the effects of a new therapy, or combine various data sources to uncover new insights to transplant patient’s journey” says Wida Cherikh, principal research scientist at UNOS.
What is a registry?
At its core, a registry is a data capture system which merges similar data from various sources. UNOS’s secure registries focus on improving patient care, sharing collaborative knowledge, and advancing understanding in the field of organ transplantation. Data from registries addresses things such as treatment, quality improvement, benchmarking, and clinical research.
In UNOS’s clinical work, the data is structured, validated, and uses real-world patient populations. Registry-based studies are quicker and less expensive than clinical trials or prospective cohort studies and can provide answers to questions that may not be answerable from OPTN data alone. Our registries scale in a variety of ways spanning the months of a specific project or study, to decades which build the backbone of research in specific fields of medicine.
What are registries used for?
Registries are used by organ procurement organizations, transplant centers, academic research centers, medical device companies, pharmaceutical companies, biotech companies and pharmacy management to capture data, manage its use, and leverage the data for insights to include patient population trends and improved outcome measures.
The transplant community relies on UNOS to build and host registries and databases, including:
- patient-level registries for large international societies
- performance improvement and outcome registries for transplant programs
- survey registries for submitting patient data
- clinical registries using observational study methodology to collect uniform data and evaluate specified outcomes for a population
Build your registry studies effectively with UNOS Solutions
The UNOS Solutions team creates registry partnerships that optimize the use of new data sources to understand outcomes in transplantation. “TransMedics partnered exclusively with our trusted partners at UNOS to create the first Thoracic Organ Perfusion (TOP) Registry for our Organ Care System (OCS) technology,” said Dr. Waleed Hassanein, President and CEO at TransMedics, Inc. “ The UNOS team has provided a comprehensive platform that delivers the visibility and compliance that is necessary to conduct our registry. Their team is world-class and provides an exceptional level of cooperation and partnership required for successful execution of these complex projects.”
Our team of research scientists, statisticians, and data analysts help you understand your data holistically, visualize your data, and give it context for analysis. You’ll be able to get reliable data, with an intuitive user interface, and receive real-time reports that allow you to see trends.
UNOS can help you find answers to your transplant-related challenges – we have the systems and people in place to create and implement registry-based studies for the transplant community.
Learn more about registries by contacting UNOS.
In focus
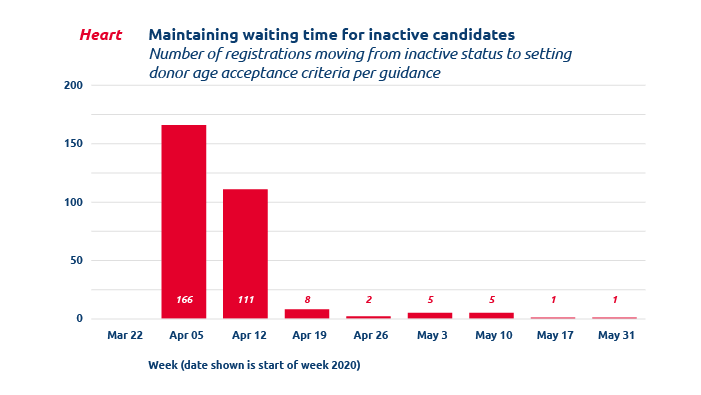
Preserving candidate wait time during temporary inactivation due to COVID-19
Altering organ acceptance criteria protects candidates at risk of COVID-19 from losing accrued wait time.
OPO testing of deceased donors for COVID-19 ensures patient safety
Recent report shows 100 percent COVID-19 donor testing by OPOs.
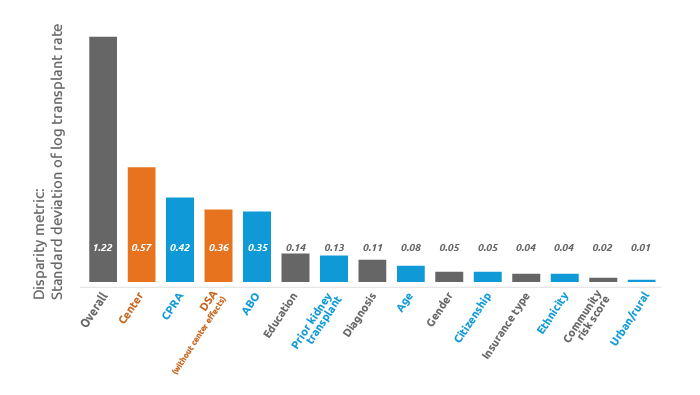
Center effects greatly affect kidney access disparity
Research shows center effects are the top driver of kidney access disparities, but DSA effects still remain
The post appeared first on UNOS.
Preliminary results of the 2018 Organ Procurement and Transplantation Network modified adult heart allocation revision show the policy is achieving its intended goals.
United Network for Organ Sharing research led by research science manager Rebecca Goff, Ph.D., analyzed OPTN data, including early outcomes, geographical distribution, and waitlist and transplant characteristics, a year before, and a year after policy implementation.
“The manuscript revealed the benefit of the new six adult heart statuses that better stratify candidates and give broader access to the most medically urgent patients,” Goff said. “Thinking to the future, I am looking forward to the development of a sophisticated heart allocation score and, eventually, the move to the continuous distribution framework for heart allocation.”
Read more about the research into the effectiveness of the new adult heart allocation policy.
The post appeared first on UNOS.
As an organ procurement trainer at the Center for Organ Recovery and Education, Nicole Medwick is focused on making sure that the right organ reaches the right patient at the right time.
“At CORE we are always looking for new and innovative ways to improve our key processes,” she said.
So when CORE’s involvement in a medical imaging study sharing project led by United Network for Organ Sharing prompted a transplant team to accept a heart that they otherwise had planned to decline, Medwick felt proud of the work she had accomplished. “We were able to save an additional life that would not have been saved otherwise,” she said.
CORE is one of 13 organ procurement organizations (OPOs) across the country enrolled in UNet Image Sharing, a consistent, reliable and secure image sharing platform that provides OPOs and transplant hospitals universal access to high-quality medical imaging studies during the organ offer process. “UNet Image Sharing helps us share real-time images directly with transplant surgeons to help facilitate quicker decision making,” Medwick said.
Hear more about how UNet Image Sharing is helping to facilitate quicker decision making and improving efficiencies in the organ procurement process for Medwick and other members of the organ donation and transplant community.
The post appeared first on UNOS.

Performance
We’ve got the best organ donation and transplant system in the world.
Here’s how to make it even better.
And we’re getting better all the time. Today, American surgeons perform 40 percent more transplants than they did eight years ago. And even in a pandemic year, the system successfully performed 500 more deceased donor transplants from January through July 2020 than it did in the first seven months of 2019. Many other countries, by contrast, had steep drop-offs in the number of transplants performed this year.
Organ transplantation works for people who have end-stage organ failure. In almost all cases, it works better for people with kidney, liver, and lung failure than any other treatment available. People come to the organ donation waiting list because they know an organ transplant is a gift of life. That means that as good as our system is, it needs to be even better to be able to serve the more than 100,000 people waiting for organs at any given time.
At UNOS, the non-profit network that leads and coordinates the nation’s system of transplant hospitals, organ procurement organizations, and thousands of volunteers from the donation and transplant community, we’re not content with the status quo. Our commitment to continuous improvement has driven seven consecutive years of increases in the number of transplants performed. We also know there’s no quick fix to further strengthen a mature system with decades of experience and an infrastructure that covers the entire nation. And because we have a full view of how every step of donation and transplant works, we know that what we need now is a comprehensive set of reforms.
Earlier this year, the Centers for Medicare and Medicaid Services proposed changing the metrics by which OPOS are assessed. The proposal is well-intentioned and addresses many issues identified by the donation and transplant community. But it relies on faulty data and has met strong opposition from major stakeholders in the professional community — including the American Society of Transplantation, Association of Organ Procurement Organizations, and a number of individual transplant programs and OPOs.
And with a narrow focus on OPO metrics, CMS’ proposal does not take a broad enough look at the systemic approach that is necessary to continue to increase the number of transplants.
These reforms would build upon our success as one of the leading systems in the world and save even more lives than ever before.
First, we need to automate real-time donor referral. This is a step we could not have proposed just five years ago. But today, hospital electronic health records give us an opportunity to have comprehensive, timely data about all potential donors. We believe we can build on that momentum and take the responsibility for referring donor candidates off the already full plates of hospital staff, who in most cases have to enter referrals manually.
UNOS and others are working to standardize a referral system that can be used by all U.S. electronic medical records providers, because automating donor referral will not only allow OPOs to identify more donors whose organs can be more quickly matched with more patients. It will also enable us to establish better metrics for OPOs and transplant hospitals.
Holding OPOs and transplants hospitals accountable for their performance and helping them to improve and save more lives requires consistent, reliable and timely metrics.
To better measure OPO performance, CMS has proposed a new metric that relies on death certificate data collected by the Centers for Disease Control and Prevention. But the CDC itself has acknowledged death certificates are not consistently accurate. They also lack the level of clinical detail OPOs, surgeons and patients need to determine donor suitability. For example, a death certificate might not tell us if the potential donor died on a ventilator or whether they had a disease that could put the transplant patient at risk. When only about 1% of people die in a way that makes them medically eligible for organ donation, having detailed information is crucial.
Automated donor referrals, on the other hand, would provide an improved, independently-reported and timely data source for understanding donor potential. This hospital-reported, patient-level data could be used to calculate a clear metric that CMS could use to assess and improve OPOs.
We also need to improve the process of getting the right organ to the right patient at the right time.
Organs sometimes go unused because the unique medical circumstances of the donor limit the number of candidates who could benefit from the organ. We’re working on a number of innovative projects to increase OPOs’ ability to place as many organs as possible, such as:
- Incorporating digital medical imaging of the donor into organ offers.
- Giving transplant programs the ability to set more sophisticated screening criteria for offers they will accept.
- Assessing probability models that may guide transplant program decisions based on their patterns of organ usage.
Similarly, we need to remove disincentives to using older and more complex donors when appropriate. Not every transplant candidate needs organs with the same projected life span, and one key step toward increasing organ transplantation is to use organs from older donors, which can be a good choice for some transplant patients, especially those who are older themselves. Unfortunately, today those organs are often rejected by transplant hospitals because they are more complex to work with — and by patients, who may not understand the viability of older organs.
We believe eliminating evaluation and financial disincentives for using these organs will help boost the number of transplants and save more lives.
Finally, we need to enable OPOs to merge or share services to boost their ability to serve hospitals and patients. CMS’ proposed rule provides no detailed transition plan addressing infrastructure in the service area of a decertified OPO, which could lead to ongoing disruption in parts of the country. Replacement organizations would need to rebuild the relationships and public trust necessary to maximize local organ procurement and distribution. Regulations that expedite or incentivize voluntary mergers among OPOs could expand the reach of effective OPO leadership without creating the risk of gaps in the organ recovery network.
There are thousands of things that each OPO, each transplant hospital, each staffer, UNOS, and CMS can do to drive improvement, but we’re targeting the things that we think apply to the whole system and that no individual part can do on their own. Taken together, these reforms will increase the pool of donors and organs, lead to shorter wait times and better outcomes, and mean more people receive lifesaving transplants. I’m excited by the potential for change, because as proud as we at UNOS are of the success of the organ procurement and transplant system, we know it has to keep getting better.
To learn more about the improvements we’re proposing, read the overview

To learn more about the improvements we’re proposing, read the overview
Read more
Getting to yes
Offer filters
Bridging the gap
The post appeared first on UNOS.

INNOVATION
As an organ procurement trainer at the Center for Organ Recovery and Education, Nicole Medwick is focused on making sure that the right organ reaches the right patient at the right time.
“At CORE we are always looking for new and innovative ways to improve our key processes,” she said.
So when CORE’s involvement in a medical imaging study sharing project led by United Network for Organ Sharing prompted a transplant team to accept a heart that they otherwise had planned to decline, Medwick felt proud of the work she had accomplished. “We were able to save an additional life that would not have been saved otherwise,” she said.
CORE is one of 13 organ procurement organizations (OPOs) across the country enrolled in UNet Image Sharing, a consistent, reliable and secure image sharing platform that provides OPOs and transplant hospitals universal access to high-quality medical imaging studies during the organ offer process. “UNet Image Sharing helps us share real-time images directly with transplant surgeons to help facilitate quicker decision making,” Medwick said.
First national hub
Within a year of enrolling in the pilot in 2019, her OPO was notified of an offer for a deceased donor’s heart that included a medical report saying the organ was functioning poorly. “Based on the report that we had, no one would have been likely to accept the heart,” Medwick said, but by using UNet Image Sharing, she was able to include an electrocardiogram with the offer. The transplant team analyzed the images and determined they didn’t agree with the report, ultimately deciding the heart would be suitable for transplant. “The transplant hospital staff only accepted it because they were able to look at the images and get their own read,” Medwick said.
More lifesaving organs being accepted and transplanted
As the first national donor imaging sharing hub, UNet Image Sharing facilitates more rapid and efficient consideration of organ offers, which can lead to more lifesaving organs being accepted and transplanted.
“UNet Image sharing streamlines the process for reviewing medical imaging studies and helps us reduce inefficiencies and the need for multiple systems,” said UNOS service owner Randall Fenderson, who has been leading the application development process and piloting the new service with members for more than two years. “As a community, we are accelerating the organ acceptance process and enabling transplant surgeons to make better, more informed decisions.”
UNet Image Sharing was created to solve a problem: If a surgeon can’t clearly see the medical imaging study of an organ that is being offered, they might hesitate to accept some offers for fear that it might not meet their patient’s needs. While well-intentioned, this risk-avoidant behavior could result in unused organs that could otherwise help save patients’ lives.
“Having images uploaded directly into UNet allows easy access for all parties involved in reviewing the quality of the organ for transplantation,” said CORE chief information officer Bruno Mastroianni. The former chair of the Association of Organ Procurement Organizations IT Council heard about the pilot in 2018 at an AOPO conference. In conversation with UNOS chief technology officer Alex Tulchinsky and IT Customer Advocacy department director Amy Putnam, Mastroianni discovered that UNOS was planning to pilot a new technology in response to members’ longstanding requests for a consistent and secure high-quality imaging sharing system that is seamlessly woven into the DonorNet platform. “I have been fortunate to have built a wonderful relationship with Alex, Amy and the rest of the UNOS team, and have volunteered to support them to pilot solutions like this before,” Mastroianni said. “It is a great opportunity to provide input and even better to work with a great partner like UNOS.”
Imaging within UNet
Between January and April 2019, UNOS piloted UNet Image Sharing with six OPOs across the country that were already using imaging solutions outside of DonorNet. “We picked OPOs that already had imaging solutions so that if something went wrong in the pilot, they still had a fallback plan that they could work with,” said UNOS business architect Rob McTier.
UNOS works with the community to improve the national system
All of the application’s functionality is available to users on the donor record in DonorNet, alongside all the other donor information. During the pilot, OPOs received high-quality imaging studies on CDs or thumb drives. They then logged into DonorNet® to upload the imaging study similar to the way they upload smaller attachments. DonorNet linked the imaging studies to a specific donor’s record. Transplant hospitals then logged into DonorNet to view the imaging study using a Digital Imaging and Communications in Medicine (DICOM®) viewing tool.
“The experience of working with UNOS was wonderful,” Mastroianni said of CORE’s participation in the pilot. “UNOS staff listened to our input carefully to deploy multiple iterations of the solution. The first version of UNet Image Sharing was good, but by listening to the pilot participants’ feedback, UNOS ensured that access worked for the right individuals needing to use the application.”
video
Faster, more informed decisions
UNOS service owner Randall Fenderson has been leading the application development process and piloting the new service with members for the past two years.
“As a community, we are accelerating the organ acceptance process and enabling transplant surgeons to make better, more informed decisions.”
Randall Fenderson, UNOS service owner
Expanding beyond the pilot
In June UNOS began making UNet Image Sharing available in a phased national rollout, first to OPOs that expressed interest in it during the pilot period, followed by OPOs in similar geographic areas to those that expressed interest. “That way, transplant hospitals are seeing a consistent way of accessing imaging studies during the transition period,” McTier said. The phased rollout also allows time for training the hospital staff that the OPOs will be sharing imaging studies with. Because of its unique position and ability to integrate all aspects of the organ transplantation system, UNOS will manage the potential impact to support personnel as new users engage with the application. There is no additional cost to Organ Procurement and Transplantation Network members.
Nearly two years since launching UNet Image Sharing, there has been a net increase in image sharing in UNet for each participating OPO, and overall. On average, 41 percent of donors from participating OPOs had images shared before the pilot started, compared to 82 percent after UNet Image Sharing began. The most uploaded type of images to UNet Image Sharing have been CT scans, followed by ultrasounds.
“There are fewer steps in the process of uploading images directly to UNet, compared to what needed to be done with our previous application,” Medwick said, adding that CORE now rarely used the external image sharing solution that they had been relying on before UNet Image Sharing was available.
In organ donation and transplantation, every second counts, and efficiency in the system leads to more lives being saved.
“UNet Image Sharing provides a consistent way for all member organizations to access images,” McTier said. “With a consistent approach, we’re hoping to accelerate the process of getting members access to images and save more lives together.”
OPOs participating in UNet Image Sharing
- Center for Organ Recovery and Education, Pittsburgh
- Donor Network West, San Ramon, California
- Lifesharing: A Donate Life Organization, San Diego
- LifeQuest Organ Recovery Services, Gainesville, Florida
- Nevada Donor Network, Las Vegas
- One Legacy, Los Angeles
- Gift of Life Donor Program, Philadelphia
- Washington Regional Transplant Community, Falls Church, Virginia
- OurLegacy, Maitland, Florida
- Donor Network of Arizona, Phoenix
- Lifeline of Ohio, Columbus, Ohio
- LifeChoice Donor Services, Waltham, Massachusetts
- New England Organ Bank, Waltham, Massachusetts
Image uploads
as of Sept. 6, 2020
3,366
CT scans
(49.2% of total uploads)
1,755
ultrasounds
(25.7% of total uploads)
The post appeared first on UNOS.
To match clinical outcomes of heart transplantation against histopathological and ultrastructural characteristics of marginal grafts preserved by cold storage or ex vivo normothermic perfusion.
The Organ Procurement and Transplantation Network is offering 10 items for public comment beginning Aug. 4.
We encourage patients, transplant candidates and recipients, living donors, donor families and transplant professionals to learn more about the proposals and provide valuable feedback to help shape U.S. organ transplant policy. To make the nation’s organ donation and transplantation system fair and equitable for all, many voices are needed and every view matters.
Learn more about the policy development process.
The items available for public comment this cycle are:
- Align OPTN Policy with U.S. Public Health Service Guideline, 2020
- COVID-19 Emergency Policies and Data Collection
- Further Enhancements to the National Liver Review Board
- Guidance Addressing the Use of Pediatric Heart Exceptions
- Guidance and Policy Addressing Adult Heart Allocation
- Modify Data Collection on Living VCA Donors
- Modify Living Donation Policy to Include Living VCA Donors
- Programming VCA Allocation in UNet
- Update on the Continuous Distribution of Organs Project
- Updated Cohort for Calculation of the Lung Allocation Score (LAS)
Educational resources, including recorded videos and presentations, will be made available Aug. 13 to provide multiple opportunities to learn more about the proposals.
Public comment closes Oct 1. All comments received about a proposed change are reviewed before the OPTN Board of Directors vote at their December 2020 meeting.
Learn about more ways to get involved.
The post appeared first on UNOS.
As one of the 58 organ procurement organizations that serve the country’s transplant system, LifeShare of Oklahoma has helped drive a record- increase in organ donation. In 2019, OPOs recovered nearly 12,000 deceased donors, an increase of 38 percent since 2014. During that same time period, LifeShare of Oklahoma increased recoveries of deceased donors in their donation service area by 39.7 percent, exceeding the national average, according to OPTN data
While the COVID-19 crisis has impacted organ recovery and transplant, OPOs are adapting and continuing their lifesaving work. In a recent interview, LifeShare of Oklahoma president and CEO Jeff Orlowski discussed how his organization’s core values keep his team focused on the work of saving lives during the pandemic and beyond.
Q: LifeShare of Oklahoma’s core values are centered on valuing a can do attitude, having passion for the mission of saving lives and caring for teammates and the community. Your strategic anchors are people, change and relationships. How do these concepts drive your organizational approach?
We are a people-first organization. That is our guiding principle.
Our donors are people, our donor families are people, our hospitals are people, and our team are people. And so we always want to make sure that we’re making decisions in a way that will have a positive impact on people.
When I came to LifeShare, we had a tremendous amount of development to do as an organization. I asked everybody to not be intimidated by change, but instead to embrace it. Bringing change is not an indictment of what came before, it’s an acknowledgement that there is opportunity to do better. We’ve woven that theme of change through everything we’ve done for the last 8 1/2 years.
Q: What does change mean as a core value in relation to transplant?
We work in a field where change is natural. It’s happening around us constantly. There will always be new drugs, new techniques and new opportunities.
The HOPE Act is a great example. There used to be a ban on HIV positive donors—and now there’s not. I always point to the fact that in 1953, you couldn’t have a kidney transplant, and here we are less than 70 years later, and it’s been a common therapy for decades. Even more recently, when I started in the field, isolated lung transplants were just not feasible for a variety of reasons—it’s only been in the last really 30 or so years that isolated lung transplants, as opposed to heart-lung blocs have become a really viable operation. Since change happens anyway, embracing it allows us as an organization to talk about what’s possible.
All change is the result of many people working together to adapt and improve. Having change as a strategic anchor has been so important in this crisis.
Q: How have your organization’s core values helped guide LifeShare of Oklahoma through the current crisis?
Our core value of can do has served us very well. Can do is not just do it. We’re not talking about being Nike. Can do is about the fact that we’re creative. When we look at a challenge, we’ll look for alternative ways to get to the desired outcome.
In terms of the pandemic, the concept of can do has been particularly instrumental in making sure our staff were safe while we keep doing the important work of recovering donors. Protecting both staff and our mission have driven us from the very beginning. When we focused on that, we were unencumbered by any other details. We just worried about getting the job done safely. When you do that, you can be more creative and kind of let go of your preconceived limitations on yourself.
We have 135 staff between our Oklahoma City and Tulsa offices, and during this crisis we have had to lean into real change as an organization—with amazing results.
Q: What are some of the changes your executive team made to adapt in response to COVID-19?
That first weekend of March 13, we started mainly trying to get our clinical staff converted to telecommute so they wouldn’t cross-contaminate one another. Their safety was a priority in those early discussions, but we didn’t really understand then what we were up against compared to what we knew just a few weeks later.
After that I took a step back and started assessing the global organizational impact. That was when staff safety and well-being started to become the guiding principle, and it was pretty easy to see how we could keep doing our mission.
Q: What do you think will be some of the permanent changes that result from the crisis?
I think one of the silver linings is that it has forced the community to rethink the concept of jumping on a jet in the middle of the night, flying a couple hours and riding around in the back of an ambulance to accomplish something that can be accomplished without everyone traveling. That’s not just OPO staff—our transplant partners are rethinking letting a local recovery surgeon recover and send an organ, because that means they can also avoid flying. This is a benefit overall in terms of broader allocation—if we can avoid putting a surgeon and a perfusion staff member or surgical recovery coordinator on an airplane for two hours one direction, and then sitting around at a hospital, waiting for everything to go and then doing the case, and then two hours more on a plane back, then those people can be home tucked in bed and fresh and wide awake when it’s time to do the transplant. We keep the transplant team safe and rested, and we make maximum use of our resources so that more lives are saved.
Q: You have spoken internationally about organ donation and recovery in the United States. What are your observations about how the U.S. system adapted to COVID-19 compared to other countries?
The COVID-19 crisis has actually illustrated how effective American OPOs are.
Other countries saw donation and transplantation basically grind to a halt, which was not the case in the U.S. We had considerably higher rates of transplants than many other countries that were tremendously impacted by this, which I think is a testament to our ability to refocus and reinvent ourselves in order to keep people getting transplanted.
When we evaluate opportunities for improvement, we must acknowledge that we’re improving from a position of strength. We’re fortunate to have extremely bright and capable people who, when faced with an unprecedented pandemic, have been able to continue to save lives through donation and transplantation through a massive amount of collaboration and teamwork, on a national level. While we always need to strive for improvement, we shouldn’t underestimate the factors that make us so successful already.
Jeff Orlowski is president and CEO of LifeShare of Oklahoma, and president of the LifeShare Foundation. He currently serves as the Organ Procurement and Transplantation Network Region 4 associate councillor, and is a member of the OPTN Membership and Professional Standards Committee. A past president of the Association of Organ Procurement Organizations and Donate Life America, Orlowski has 33 years of experience in organ and tissue donation and has co-authored more than 40 peer-reviewed articles. Orlowski earned a bachelor’s degree in biology from the University of Kansas and a master’s degree in management from Regis University.
The post “Can do, passion and caring”: Jeff Orlowski on embracing change during COVID-19 appeared first on UNOS.
Despite a decade since a 2007 fatal plane crash involving a University of Michigan transplant team and pilots, there remain no federal standards regulating air and ground transportation of organ recovery personnel.

The American Society of Transplant Surgeons, the American Society of Transplantation, the Association of Organ Procurement Organizations, and United Network for Organ Sharing convened to develop national transportation standard recommendations.
In an article published in the April issue of the American Journal of Transplantation, University of Iowa kidney surgeon David Axelrod, M.D., United Network for Organ Sharing CEO Brian Shepard, and other members of the organ donation and transplant community summarized their discussions and defined national standards for organ recovery practice in three areas: air transportation, ground transportation and insurance coverage.
Recommendation highlights included:
- Expanding air transport quality assurance protocols, including a requirement for twin-engine, turbine-powered aircrafts piloted by two qualified pilots and operated by organizations that have been certified through onsite inspections
- Ensuring teams travel in dedicated vehicles with adequate safety restraints; ambulances are avoided whenever possible; and, the use of lights and sirens during transport is minimized
- Providing adequate insurance coverage for all organ recovery team members, including trainees
New organ allocation policies that mandate broader sharing have highlighted the need to formalize transportation safety protocols as an essential element of the national standards followed by all transplant teams. The organ donation and transplant community members convened in 2008 to develop standards meant to ensure the safety of transplant team members and organ procurement organization personnel who travel to recover lifesaving organs.
Axelrod DA, Shah S, Guarrera J, et al., Improving safety in organ recovery transportation: Report from the ASTS/UNOS/AST/AOPO transportation safety summit. Am J Transplant. 2020;00:1–8. doi:10.1111/ajt.15930
In focus
Analyzing adult heart allocation policy
In a recently published article, UNOS-led research found that the new heart allocation policy provides broader access to the most medically urgent candidates.
Gauging social media effectiveness in finding living donors
At the 2020 American Transplant Congress, UNOS and OPTN researchers presented insights of U.S. transplant hospitals using social media campaigns to identify potential living donors.
Policy keeps patients safe, protects medical urgency status
Staff and members collaborated to create emergency policy and IT changes to protect patients and keep the system nimble during the COVID-19 pandemic.
The post appeared first on UNOS.
UNOS collaborated with nine global transplant organizations and societies on four COVID-19 organ donation and transplant town hall webinars that have received more than 25,000 views.
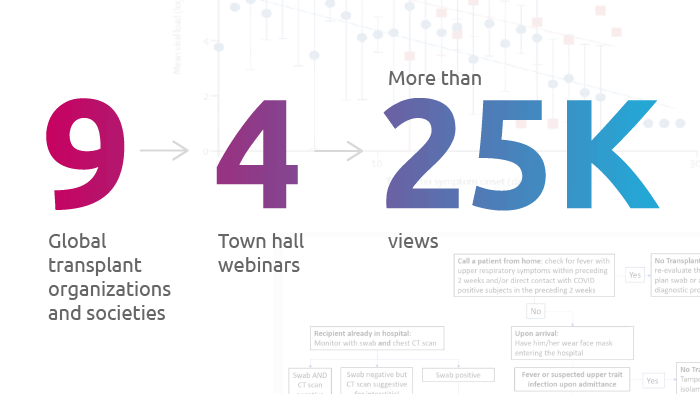
Organ donation and transplantation organizations acted swiftly to produce town hall-style webinars.
As COVID-19 cases began increasing globally, conversations among health care providers about the pandemic’s potential impact on transplant started to emerge.
Within a few days, organ donation and transplant communities from around the world assembled to collaborate on a webinar series sharing real-world, global experiences for the organ donation and transplant community. Along with United Network for Organ Sharing, participating organizations included:
- American Society of Transplantation
- Association of Organ Procurement Organizations
- American Society of Transplant Surgeons
- North American Transplant Coordinators Organization
- United Network for Organ Sharing
- Canadian Society of Transplantation
- European Society of Transplantation
- International Society for Heart and Lung Transplantation
- The Transplant Society
The societies launched their first live, collaborative webinar “COVID-19: Organ Donation and Transplant Town Hall,” on March 23, and the 1,000 available seats filled up within a few hours. Collaboration continued over the following weeks with three additional webinars, covering topics ranging from:
- Presentation, diagnosis, treatment and prevention
- Getting to transplant
- Operational issues
- Screening donors and candidates
- Protecting the workforce
- Ethical issues
The societies aren’t done yet, with future plans to produce more collaborative webinars on navigating COVID-19, as well as topics unrelated to the pandemic.
Read more about the COVID-19 pandemic and collaboration within the transplant community.
In focus
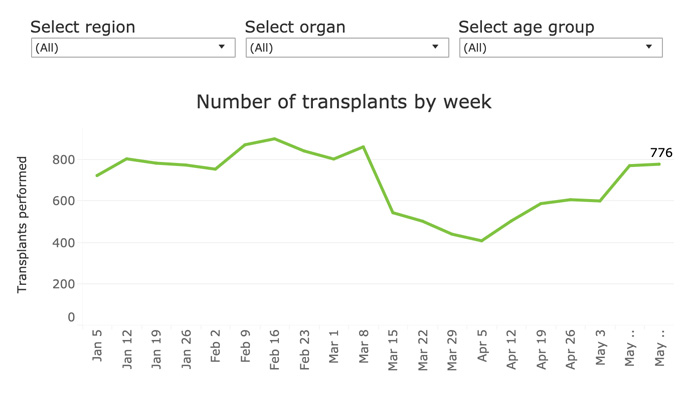
Transplants bounce back to near pre-COVID-19 levels
Monitoring transplants during the COVID-19 pandemic, the UNOS data visualization shows changes in weekly organ transplants.
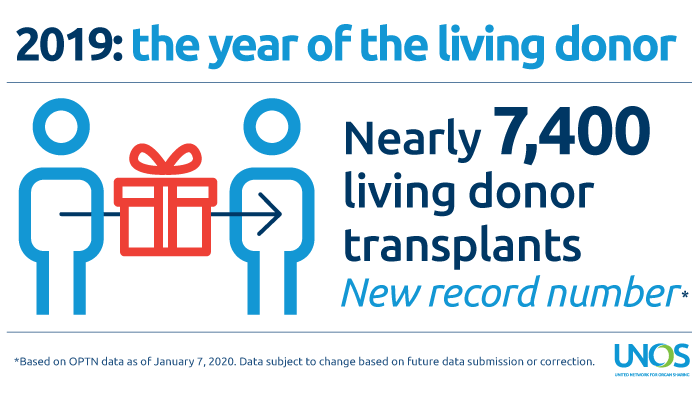
2019: Record-setting year for living donation
Education has become an essential tool transplant programs are using to increase understanding and awareness of the procedure.

Is natural language processing useful in predicting organ acceptance?
UNOS data scientists explore donor admission text to help understand and predict how kidney acceptance decisions are made
The post appeared first on UNOS.
A Q&A with American Society of Transplantation immediate-past president Emily Blumberg, M.D.
As COVID-19 cases began increasing globally, conversations among health care providers about the pandemic’s potential impact on transplant started to emerge. “This sense of the need to educate our communities—both individually and potentially as a group—came up, and we started sending around emails amongst ourselves,” said transplant infectious disease specialist Emily Blumberg, M.D., who recently served as president of the American Society of Transplantation.
Within a few days, organ donation and transplant professionals from around the world had assembled to collaborate on a webinar series sharing real-world, global experiences for the organ donation and transplant community.
“COVID-19 presented a unique opportunity for us to convene not only transplant associations in the United States, but across the world,” said former UNOS Board of Directors president Stuart Sweet, M.D., Ph.D., who recently concluded his term as president of the International Society for Heart and Lung Transplantation. “It was really taking advantage of a challenging opportunity to convene our community in a way that we’d never done before.”
Registration for the first live, collaborative webinar “COVID-19: Organ Donation and Transplant Town Hall,” launched on March 23 and the 1,000 available seats filled within a few hours. Collaboration continued over the following weeks, with three additional webinars covering topics ranging from protecting the workforce to conversations around testing. Collectively, the webinar recordings have been viewed more than 25,000 times.
In a recent interview, Blumberg talked about how the organ donation and transplantation community united amidst the COVID-19 pandemic to launch the webinar series.
How did the idea for the collaborative COVID webinars come to be?
I’ve been thinking back about how this started and honestly, I think there were a lot of side conversations that happened among leadership of these different organizations. But somehow, organically, this sense of the need to educate our communities—both individually and as a group—came up, and we started sending around emails amongst ourselves. All of a sudden we had a group of committed individuals who thought that we should try to share information in real time. There was tremendous brainstorming about speakers at every juncture and how to make it international. I think people were just really interested in helping to meet the challenge of COVID with information to the best of our ability in real time.
The town hall webinars are a collaborative effort between:
- American Society of Transplantation (AST)
- Association of Organ Procurement Organizations (AOPO)
- American Society of Transplant Surgeons (ASTS)
- North American Transplant Coordinators Organization (NATCO)
- United Network for Organ Sharing (UNOS)
- Canadian Society of Transplantation (CST)
- European Society of Transplantation (ESOT)
- International Society for Heart and Lung Transplantation (ISHLT)
- The Transplant Society (TTS)
What was the goal?
The first goal was to disseminate current information from people who were already experiencing the management of transplant patients with COVID-19. We wanted to have people who could give us real world experiences and who would have a perspective to help pave a way for everybody else to start thinking about it. One of the incredible things about the webinar was having the international components provided by several of the societies. Especially with everything that has gone on in Italy, ahead of us, in terms of experience. We could actually engage people who knew a little bit more about what they were seeing and how they were handling it and get some real-time, practical information. I think this is something that would never have been doable if we had tried to stay local.
How did everything come together?
On our first call, we were brainstorming about different topics that people were interested in hearing about and that we thought would be helpful, and where different members of the societies were experience-wise. We made an enormous list of these. Then we pared the list down and tried to make it into a cohesive whole. We really benefited a lot from UNOS their willingness to use their platform. Because UNOS had the technology already set up, that became an immediate weight off everybody’s shoulders about how we were going to do this. It also took out of any individual society’s purview, which I think helps the collaboration and made it easier. We asked everyone to come in with three really relevant slides and five minutes of critical information. It really focused the individuals and allowed us to bring many more viewpoints.
What was the experience like working with so many societies?
I don’t think anybody, at any point, saw this as owned by any individual society. I think we all appreciated what all the different societies had to contribute and their commitment to it. It’s an extraordinary group effort. Personally, I knew some of the people involved in advance of the webinars, but there were people I’ve met through this experience who it’s been such a pleasure to collaborate with. I think that could be said for all of us.
What surprised you most about the collaboration?
I’ve been impressed by how engaged people have stayed throughout the entire process. It wasn’t like the first one was done and everybody checked out. We realized people were really interested and it made people engaged in doing another one. When we did the second one and the extra module for it, we said, “Wow, people are still interested.”
How do you think this collaboration has helped the transplant community as they navigate COVID-19?
We’re all benefiting from learning from everybody’s experience for sure. I mean, there have been some difficult times for all of us. Having this information to fall back on has been really beneficial. Just in our day-to-day jobs in taking care of patients, setting up things in our various transplant hospitals and trying to learn from people’s experience so you’re not starting at the bottom. You’re actually starting with the foundation that you’ve gained from interfacing with people throughout the world.
What do you hope the community has gained from these events?
I think we’ve gotten to know one another a little better and feel more globally joined as a community, in a way that maybe we didn’t feel so much before. The societies have interacted one-on-one with other societies on certain initiatives, but this experience let us know that people are interested in a bigger world. Not that the societies individually are going away, but that they’re finding new ways to collaborate and we’re all learning from one another.
What’s your hope for the future?
I’ve talked individually with some of the other leaders of other societies and I think we would like to try to continue some level of international education. I hope that we’re going to be able to talk in the next week or two to figure out what our next steps are going to be. I think it would be nice to expand this tent to include more people.
This was one of the most exciting things that I’ve gotten to participate in during this past year. I would have never foreseen the incredible positive force that such a collaboration could generate. I think it all made us feel we were doing something important for the community. We all had a common goal and a common interest and it was really a pleasure.
The post appeared first on UNOS.
Patient Q&A with UNOS Chief Medical Officer David Klassen
Navigating the coronavirus pandemic is difficult for everyone, but brings special challenges for transplant patients. In a new video series on TransplantLiving.org, UNOS Chief Medical Officer David Klassen, M.D., answers questions for patients about COVID-19 and the waiting list, temporary inactivation, donor testing, living donation and other important topics.
Reactivation process for multiple kidney transplant candidates now available
As of April 22, kidney transplant programs are now able to simultaneously perform reactivation of multiple candidates whose current status is “Temporarily Inactive (7),” with inactive reason “COVID-19 Precaution.” Details and directions were sent via email to staff at all kidney transplant programs and posted on Secure Enterprise. In addition, step-by-step instructions are available in online help in UNetSM.
Programs also have the ability to perform temporary inactivation of multiple transplant candidates simultaneously for the reason of “COVID-19 Precaution,” which was released March 27. Together, this set of tools ensures offers are made efficiently to candidates who are immediately eligible for a transplant and streamlines the offer process.
Before temporarily inactivating candidates, please reference OPTN policy 3.6.A for information about whether candidates needing a particular organ type accrue waiting time while inactive.
ISHLT presents new webinar: COVID-19 and challenges in advanced heart and lung disease and cardiothoracic transplantation
The International Society for Heart and Lung Transplantation is holding a free, live webinar for the cardiothoracic transplant community on April 29 at 4 p.m. ET. A recording will be posted for later viewing. Membership is not required and CE credit is available.
UNOS Staffing Survey deadline extended through Dec. 31
The deadline for the UNOS 2019 staffing survey has been extended from May 31 to Dec. 31. The extension is in response to the COVID-19 pandemic and will ensure maximum participation from as many hospitals as possible.
Reminders
COVID Collaborative now open for registration
The OPTN launched an interactive forum on April 22 so that members may engage on topics specific to donation and transplant during COVID-19. The OPTN COVID Collaborative operates on a secure online communication platform and allows donation and transplant professionals to collaboratively discuss, organize, catalog and archive the learnings and insights developed during the 2020 pandemic.
The site will:
- Offer discussion forums, facilitated by collaborative improvement specialists
- House and organize the member-driven information that results from this collaboration
- Be available for the duration of COVID-19, or until the decision is made to close the project
- Deploy national and regional webinars to support the spread of effective practices that emerge from site discussion
Participation in the COVID Collaborative is limited to individuals from OPTN member organizations. Registration is required. The scope of discussions will include OPTN member business and administrative processes related to the management of COVID crisis, and discussions will be moderated. Members with questions about policy during the pandemic should email member.questions@unos.org. Members with questions about policy compliance during the pandemic should email MQFeedback@unos.org.
Members may register here. Participants will receive an email with account and login information within two business days of registering.
For more information about the OPTN COVID Collaborative, email covidcollab@unos.org
Temporary changes to transplant program member monitoring as a result of the pandemic
MPSC leadership has evaluated specific areas of monitoring that are raising particular concern within the community. In an effort to encourage transplant programs to use their clinical judgement about what is in the best interests of their patients without the added concern of strict compliance with OPTN obligations, the MPSC will implement several time-limited emergency changes to member monitoring. These include:
- Suspending functional inactivity reviews through Sept. 30
- Placing a temporary hold on reviews of patient notification of extended waiting list inactivity and transplant program inactivation through Sept. 30
All time-limited COVID-related updates to monitoring changes may be found in a supplement to the OPTN member evaluation plan on the OPTN Compliance page. This document serves as a quick-reference guide to all member monitoring changes implemented as a result of the pandemic. It will be regularly updated with any additional monitoring changes implemented during this event.
Site surveys
Site surveys will be conducted virtually at least through the end of June. Any member who is not able to participate in a survey at this time can request a delay by emailing MQFeedback@UNOS.org
Patient safety portal reporting
Remember to report any suspected transplant-related disease transmissions through the Patient Safety Portal. At this time, the major transplant societies (AST, ASTS, AOPO, TTS) recommend against transplanting organs from a donor who has tested positive for COVID-19 from a respiratory tract sample or the blood, as this is likely indicative of an active infection that could be transmitted to a recipient, organ procurement team, transplant team or other contact.
New resources
- Challenges in Heart Transplantation in the Era of COVID-19: Journal of the American Heart Association
- Morbidity and Mortality Weekly Report (MMWR): COVID-19: Centers for Disease Control and Prevention
Stay current
We will continue to update the UNOS COVID-19 resources page as guidance is developed and enhanced.
The post appeared first on UNOS.
Audience
- OPO directors, administrators, data coordinators and clinical coordinators
- Please share this notice with anyone in your organization who would benefit from this information.
Implementation date: April 6, 2020
Updates for electronic organ offer notifications provide additional efficiency and flexibility about how to offer organs
An update has been made for OPO electronic organ offer notification limits in DonorNet® to provide additional efficiency and flexibility to make informed decisions about how to offer organs using their own defined best practices. Revised heart and lung policies no longer use the donation service area (DSA) as the first unit of allocation, and therefore candidates at the top of the match runs may include potential recipients who are beyond the DSA border.
What you need to do
Directions were included in an e-mail sent April 6 to all OPOs and are posted in a system notice in Secure Enterprise.
Additional resources
DonorNet® online help documentation has been updated so that OPOs will have access to the information about how local limits are used for heart, heart/lung, lung and liver matches.
Questions?
If you have questions relating to implementation, contact UNOS Customer Service at unethelpdesk@unos.org, or call 800-978-4334 from 8 am to 7 pm EST.
For policy-related questions, contact member.questions@unos.org or call 844-395-4428.
The Organ Center is available around the clock and can be reached at 800-292-9537.
The UNOS COVID-19 resources page is refreshed regularly with the most current updates and resources. Please check back frequently for updates
The post appeared first on UNOS.
UNOS, ASTS, AOPO and AST all urge local recovery of organs
The donation and transplant community has expressed unified support for the surgical recovery of organs by local teams. UNOS, ASTS, AOPO and AST released statements regarding this important step in reducing exposure to COVID-19, safeguarding the health of clinicians and facilitating the continuation of organ transplant in this challenging environment.
UNOS
“To protect the health and safety of all involved in organ recovery and transplantation during the COVID-19 pandemic, UNOS urges members to arrange for the surgical recovery of organs by local teams whenever possible, including by recovery surgeons who may be available within the donor hospital.”
ASTS
“The best way to prevent COVID-19 spread by a donor organ recovery team is to AVOID TRAVEL BY THE TEAM. ASTS strongly recommends that local teams be requested to recover organs and ship them to the recipient center for transplantation.”
AOPO
“AOPO urges OPOs to arrange for the surgical recovery of organs by local teams whenever possible consistent with the recent [UNOS] statement and the CMS requirements for the approval of recovery surgeons and the AOPO Standard for Surgeon Qualifications. This would include prioritizing the use of recovery surgeons that may be available within the donor hospital.”
AST
“[AST] strongly believe[s] that it is in the best interest of organ donation and transplant team members to limit the recovery of organs to local teams, unless there are extenuating reasons for the transplanting team to perform the recovery.”
New option to report cause of death for transplant candidates and recipients
A new cause of death code, “Infection: Viral – Covid-19,” has been added to WaitlistSM and TIEDI® to allow transplant program staff to more accurately report data. This new code will allow for better assessment of the impacts of the COVID-19 pandemic on transplant candidates and recipients. Process directions were emailed April 1 to staff at all transplant programs and posted in Secure Enterprise.
SRTR extends data review period deadline from April 30 to May 31
In light of the COVID-19 pandemic, SRTR is extending the Data Review Period deadline from April 30 to May 31. This allows transplant programs and OPOs more time to review their data for accuracy during this difficult time. Given the rapidly evolving situation, the deadline may be extended again in the future, and SRTR will work closely with HRSA and OPTN leadership to make that determination. Data Integrity Reports and program-specific report drafts for transplant programs and Donor Level Data sheets and OPO-specific report drafts for OPOs are available to review from April 1 to May 31, until 11:59 PM EDT. Verify data and make changes in UNet or DonorNet®. UNOS, the SR and HRSA are discussing modifications to the performance metrics contained in the program-specific and OPO-specific reports and will share more information as it becomes available.
New resources
- Impact of COVID-19 on organ donation and transplantation: Data visualization, United Network for Organ Sharing
- COVID-19 FAQs: For children with solid organ transplant or heart failure, Advanced Cardiac Therapies Improving Outcomes Network (ACTION)
- TTS COVID-19 Dashboard, The Transplantation Society
- Coronavirus disease 2019: Implications of emerging infections for transplantation, American Journal of Transplantation
Stay current
We will continue to update the UNOS COVID-19 resources page as guidance is developed and enhanced.
The post appeared first on UNOS.
While cardiac transplantation remains the most effective therapy for end-stage heart failure, it continues to be plagued by a severe donor shortage. Many potentially suitable organs are not transplanted due to concerns of inadequate preservation with hypothermic static storage. Experimental and clinical studies suggest that machine perfusion preservation improves myocardial preservation. The purpose of this study is to test the hypothesis that a novel hypothermic machine perfusion preservation device maintains allograft oxidative metabolism, limits lactate accumulation, and more effectively preserves high energy phosphate stores compared to cold storage in a human model.
Transmedic Organ Care System (OCS) allows preservation of the donor heart by perfusing the organ at 34°C in a beating state and providing additional assessment options. The perfusion protocols as established by the manufacturer is valid for adult human hearts, using coronary flows in the 8-900ml/min range guided by lactate measurements. Higher flow can facilitate higher degrees of cardiac oedema which can be associated with diastolic dysfunction, and generel impairment of contractility of the heart.
Ex vivo heart perfusion is an innovative preservation technique, that permits graft assessment and extended out-of-body intervals. In times of high-risk recipients and donors with extended criteria, we hypothesized that its properties for prolonged heart preservation might be especially beneficial.
Ex situ heart perfusion (ESHP) allows resuscitation and functional assessment of marginal donor hearts to expand the donor pool. The current clinically available ESHP platform does not enable contractile assessment. We sought to determine if a marginal donor heart could be resuscitated and assessed on our custom ESHP system following an extended period of cold storage.
Organ donation and transplant professionals from around the world convened on March 23 in a town hall webinar aimed at helping health care professionals navigate the ever-evolving COVID-19 health crisis. “Our goal was to share our experiences to date and respond to questions about the impact of COVID-19 on organ donation and transplantation,” said Shandie Covington, Chief Executive Officer of the American Society of Transplantation (AST) who worked with colleagues from nearly a dozen organizations to produce the free live webinar for 1,000 organ donation and transplant professionals across the country.
A recording of “COVID-19: Organ Donation and Transplant Town Hall” is now available online.
Welcome and Introduction – Emily Blumberg (AST)
COVID-19: Presentation, Diagnosis, Testing, Treatment, and Prevention – Moderator: Deepali Kumar (AST)
- Background/Disease Presentation – Erika Lease (ISHLT)
- U.S. Cases – Ajit Limaye
- Immunosuppression management in COVID+ – Attilio Iacovoni (ESOT)
- Antivirals and Immunomodulators – Paolo Grossi (ESOT)
- Prevention (infection control) – Marian Michaels (AST)
- Pediatrics – impact on this population – Lara Danziger-Isakov (ISHLT)
Getting to Transplant: Donor Issues and Candidate Concerns – Moderator: Lloyd Ratner (ASTS)
- Getting to transplant
- Donor issues/screening and testing platforms – Mike Ison (TTS)
- Procurement strategies – special safeguards needed for HCW, OPO and surgeon – Ajit Limaye, Kevin O’Connor (AOPO), Kelly Ranum
- Candidate concerns – managing the organ offer – Luciano Potena (ESOT)
Operational Issues (In-House and Managing Patient Concerns) – Moderator: Maryl Johnson (UNOS)
- Operational Issues
- Working with the institution to function during a pandemic – scaling back services, patient cohorting, logistics, determining resource sharing – Atul Humar (CST), Luciano Potena (ESOT)
- Managing patient and family concerns, 90-day medication supply – Stacee Lerret (NATCO)
- Practical approaches to visits off-site (telemedicine, etc.) – Lewis Tepermann (ASTS)
Next steps: Data Collection and Sharing – Moderator: Stuart Sweet (ISHLT)
- Next steps
- Data collection – the need to collect data and better understand what we are seeing – Tim Pruett (ASTS)
- Clearing peer review to get information into journals efficiently – Allan Kirk (AJT)
Closing and Resources: Emily Blumberg (AST)
- Available Resources
- Refreshing resources as new information is established
- Closing Thoughts
The town hall webinar was a collaborative effort between:
- American Society of Transplantation (AST)
- Association of Organ Procurement Organizations (AOPO)
- American Society of Transplant Surgeons (ASTS)
- North American Transplant Coordinators Organization (NATCO)
- United Network for Organ Sharing (UNOS)
- Canadian Society of Transplantation (CST)
- European Society of Transplantation (ESOT)
- International Society for Heart and Lung Transplantation (ISHLT)
- The Transplantation Society (TTS)
For more information about COVID-19, including advice from the Centers for Disease Control and Prevention, the Organ Procurement and Transplantation Network, and other sources, please visit UNOS’ COVID-19 resources page. The page will be refreshed regularly with the most currently available information.
The post COVID-19’s impact on organ donation and transplantation discussed at multi-society webinar appeared first on UNOS.
The Organ Procurement and Transplantation Network Board of Directors has approved replacing the OPTN Thoracic Organ Transplantation Committee with two separate committees – a Heart Transplantation Committee and a Lung Transplantation Commiteee.
The new committees will begin their work on July 1, 2020 and will allow the OPTN to be more representative and responsive to the community’s needs, which will in turn help patients.
There is an immediate need to fill a number of positions on the Heart and Lung Committees. Committee members are volunteers, representing a variety of professional disciplines, personal experience and expertise in organ transplantation.
United Network for Organ Sharing as the OPTN values diversity and strives for a Board and Committee system that represents the community we serve. Therefore, members may be donation or transplant professionals, transplant candidates or recipients, living donors, donor families, and/or members of the general public.
- If you wish to be considered for a Heart Transplantation Committee or Lung Transplantation Committee volunteer position beginning July 1, 2020, complete the online Biography Form no later than March 25, 2020.
- Please note: in the Biography Form under “area(s) of interest” you must select “Thoracic Committee” in order to be considered. Applications received after this date are not guaranteed review for the coming cycle.
- To update a previously submitted Biography Form, follow these instructions.
- To learn about volunteer responsibilities and the selection process, visit the Get Involved page.
- If you have questions about your application, please contact volunteer@unos.org.
Find information about serving on committees, and details about current openings on the Heart Transplantation Committee and the Lung Transplantation Committee on the OPTN website.
Please contact Sara Rose Wells, Transplant Community Administrator, at sararose.wells@unos.org with any questions.
The post appeared first on UNOS.
Business intelligence, or BI, is the process of transforming data into meaningful information. Some organizations use these insights to make decisions to improve performance and reduce operational costs, but in transplantation — when data literally has the power to save lives — its value is beyond measure.
The BI and data products team I lead at United Network for Organ Sharing works with the organ donation and transplantation community to build effective, efficient and user-friendly data tools that help members better navigate transplant’s many challenges. To do this, we need to have an in-depth understanding of not only data but also complex policy specific to different organs. We want to empower users to make optimal use of the information available to them.

BI and data products team (left to right) Adam Bacigalupo, Neelam Singh, Tim Baker and Read Urban.
The data tools we build provide performance indicators, operational metrics and comparison analyses that can assist transplant programs in identifying problem areas. This helps them make better decisions. A number of our efforts right now are focused toward helping transplant programs find anomalies in their data. If we help them make corrections at the right time, the data can increase a patient’s chance of finding the right organ.
We also partner with other UNOS departments like Member Quality. Connecting with and training site surveyors offers us an opportunity to cultivate other data tool learning opportunities for members, which is invaluable.
We are making improvements to existing visual analytics tools such as the kidney waiting list management tool and the living liver donor follow-up report. My team is always thinking about usability and clarifying the objectives of these tools. Even a seemingly subtle change like adding a separate documentation tab can be a significant enhancement for users.
Everyone in the donation and transplant community will tell you that managing data through information technology is at the heart of the work. It’s how people save lives every day. So it’s exciting to get the community involved in these changes — that collaboration is what helps us do our job, which is ultimately to save lives by increasing transplants.
The business intelligence team is eager to work directly with members while developing products and revamping the existing catalog. To submit feedback on any of the data portal tools, or to submit ideas for new tools, please email dataportalfeedback@unos.org.
Neelam Singh joined the UNOS Research Department in 2019 as manager of the BI and data products team. While earning her MBA in finance from Goa University in India, she had the opportunity to study abroad for a semester at Technische Hochschule, an applied sciences university in Ingolstadt, Germany. This blend of technical ability and business insight has uniquely prepared her for a career in business intelligence. She brings to UNOS more than 10 years of health care data management experience, including building and implementing quality data marts, developing business intelligence capabilities, providing data solutions, designing data process roadmaps and leading standardized reporting projects.
The post appeared first on UNOS.
New project aims to better predict organ travel time.
In the past ten years, more than 45,000 organs recovered for transplantation were not used to save lives, according to data from the Organ Procurement and Transplantation Network.
Part of the organ discard rate can be attributed to prolonged cold ischemic time, which is the time that an organ spends outside the body between procurement and transplantation. “Cold ischemic time not only limits how far an organ can travel, it is also linked with higher rates of organs being declined by transplant hospitals,” said United Network for Organ Sharing data science manager Andrew Placona. Each organ possesses its own cold ischemic time limit. While kidneys can typically survive outside the body for 24 to 36 hours, livers, intestines and pancreata only last on average for about 12 to 18 hours. Hearts and lungs have even shorter cold ischemic time limits, typically lasting outside the body for four to six hours on average.
“Speedier transportation will result in more organs transplanted, more lives saved and more improved long-term outcomes for transplant recipients,” explained Placona, who is serving as principal investigator on research that aims to help organ procurement organization, or OPO, professionals make more informed decisions about optimal travel routes for procured organs.
A joint effort
Partnering with seven OPOs across the country—along with the travel companies they enlist to help transport organs—Placona’s team is conducting real-time data analysis to refine a feasibility algorithm aimed at predicting the optimal route for organ transplantation.
Describing the algorithm as an Expedia-like application that OPO and transplant hospital professionals can use to choose the best route for an organ, Placona said he envisions people using the application to make informed decisions about optimal travel methods for organs and then tracking the organs in real time as they make their way from the donor hospital to the transplant hospital.
“This project addresses a complex problem in organ transplantation, mainly coordinating logistics for organ transport and understanding the components of cold ischemic time,” said Jeffrey Orlowski, president and CEO of LifeShare Transplant Donor Services of Oklahoma, which is the organ recovery organization for the state of Oklahoma.
Beginning December 2019, the UNOS information technology team began assessing the feasibility of building application programming interfaces that will act as bridges between the OPOs’ transportation vendor databases and UNOS’ systems to enable the flow of data between the applications. The feasibility study will continue through August 2020.
The other portion of the project involves analysis to determine if the collected data can contribute to the existing feasibility algorithm, which combines major airline and limited charter availability with current drive times to potentially determine the most efficient travel options at any given moment. The research is being conducted through UNOS LabsSM, which is an experimental incubator that unites data, technology and industry expertise to test transformational ideas and hypotheses for improving the transplant system.
“With a better understanding of how transit time impacts cold ischemic time, we could potentially assign an estimated cold ischemic time upon arrival into UNet,” explained Placona. UNetSM is UNOS’ electronic network that allows transplant professionals to register transplant candidates on the national organ waiting list, match the candidates with donated organs and enter vital medical data on candidates, donors and transplant recipients. “This would allow for more informed organ offer considerations.”
Using big data to solve complex problems
In the future, the research team plans to expand the algorithm to incorporate additional data inputs, such as weather, flight cargo limits and expanded charter flight availability. They also plan to validate the model using previously planned organ travel information, collaborating with courier services and OPOs. After validating the model, UNOS will create a user-friendly application that will enable piloting of the algorithm with more OPOs.
“In our line of work, time is critical and lives depend on timely response,” Orlowski said. “This project explores solutions to organ transportation and will help optimize travel and troubleshoot for weather, traffic and flight changes in real time.”
Placona expects the project to eventually result in reduced prolonged cold ischemic time and enable better transplant outcomes, including reducing organ discards and rejections. “Ultimately, our goal is to increase organ utilization and improve outcomes for patients,” he said. “If we can use data science to figure out where the inefficiencies are in the organ procurement process, then we can start finding solutions.”
Interested in increased efficiency?
UNOS technology specialists develop APIs that connect OPOs, transplant hospitals and histocompatibility centers so their applications can seamlessly exchange data with UNetSM applications. Learn about available APIs.
The post “An Expedia for organ transplantation” appeared first on UNOS.
The Organ Procurement and Transplantation Network is offering 11 proposals for public comment beginning on Wed., Jan. 22.
Comments and replies will be published on https://optn.transplant.hrsa.gov/governance/public-comment/, to promote transparency and trust in the national transplant system. Visitors can also share comments on social media, if they wish.
We encourage patients, transplant candidates and recipients, living donors, donor families and transplant professionals to learn more about the proposals and provide valuable feedback to help shape U.S. organ transplant policy.
These are the proposals available for public comment:
- HLA equivalency tables update 2020
- Distribution of kidneys and pancreata from Alaska
- Addressing medically urgent candidates in new kidney allocation policy
- Enhancements to the National Liver Review Board
- Data collection to assess socioeconomic status and access to transplant
- Modify blood type determination and reporting policies
- Guidance on blood type determination
- Modifications to released kidney and pancreas allocation
- National Heart Review Board for pediatrics
- Update to VCA transplant outcomes data collection
- Measuring transplant outcomes by collecting data on children born to uterus recipients
There are educational webinars available that provide everyone an opportunity to learn more about the proposals.
See webinar dates and register.
Public comment closes on March 24. All comments received about a proposed change are reviewed before the OPTN Board of Directors votes.
The post appeared first on UNOS.
Overview of Changes
On Thursday, Jan. 9, 2020, several OPTN policy updates will be implemented to eliminate the use of donation service areas, or DSAs, from heart transplantation and replace them with a 250 nautical mile circle. Additionally, the term “zone” will be removed from thoracic (heart and lung) allocation policies and replaced with the actual distances in nautical miles between donor hospital and transplant hospitals where candidates are listed. (Previous policy action in 2017 removed DSA from lung allocation). An additional changes will remove current policy language regarding prioritization of sensitized candidates within a DSA.
All of the policy updates are included in this policy notice, reflecting actions the OPTN Board of Directors approved at its June, 2019 meeting.
System Implementation Information
To implement these changes, the UNOS Secure EnterpriseSM system, which includes UNetSM and other transplant applications such as WaitlistSM, DonorNet®, TIEDI® and KPDSM, will be unavailable on Thursday, Jan. 9, from 7:00 a.m. until approximately 7:30 a.m. EST.
The policy changes do not affect definitions of “local” thoracic candidates used in:
- donor acceptance criteria within Waitlist
- contact management within DonorNet
- notification limits for organ offers
Review processes described in the OPTN Member Evaluation Plan are not affected by any of these updates.
Resource information
- A resource document, “Thoracic Allocation Using Nautical Miles,” is available in UNOS Connect from the Heart category of the course catalog, offering HRT105-Dhttps://unosconnect.unos.org/
- Online help documentation covering UNetSM functionality will be available when the system updates go into effect. Access Secure Enterprise and then choose Waitlist. On the Help menu, click Waitlist Help. You may search for a specific help topic or use the table of contents to assist with your search.
Questions?
If you have questions about the implemented updates, contact UNOS Customer Service at 800-978-4334. For policy-related questions, send an e-mail to member.questions@unos.org or call 844-395-4428.
The post appeared first on UNOS.

Pictured above: The LifePort Transporters were developed after CEO David Kravitz’s father needed a heart transplant. Photos courtesy of Organ Recovery Systems
While static cold storage remains the organ preservation standard, an alternative technology is seeking to combine its benefits with the promise of machine perfusion.
Organ Recovery Systems founder and CEO David Kravitz has more than a professional interest in transplantation. In the 1990s while the entrepreneur was running a company under contract with the U.S. military to improve battlefield trauma care, his own father ended up needing a heart transplant.
At the time Kravitz had no experience with organ transplantation, so he was astonished to learn that the heart that would save his father’s life could only come from within a few hours’ distance and would “show up in a beer cooler,” he said. “I just couldn’t quite believe it, and the doctors were telling me that was the state-of-the-art.”
Kravitz was sure there had to be a better way to protect and preserve an organ in transit. For years his efforts had been focused on trying to use interventional hypothermia — deliberately lowering a patient’s body temperature — to protect the brain from neurological complications following battlefield trauma. Drawing on this work, he conceived an interventional hypothermia-inspired design for a mechanized organ transporter. “The original vision was to enhance portability and preservation, keeping the organ alive and sustained so it could travel longer and be healthier,” he said.

Organ Recovery Systems CEO David Kravitz developed the idea for an interventional hypothermia-inspired design for a mechanized organ transporter after his father needed a heart transplant. Photo courtesy of Organ Recovery Systems.
From idea to action
A company focused on organ preservation products and services, Organ Recovery Systems was formed to develop the interventional hypothermia-inspired organ transporter concept. In the early 2000s the company’s LifePort Kidney Transporter, a transportable device for hypothermic machine perfusion, or HMP, gained Food and Drug Administration approval. Unlike normothermic preservation, which preserves organs at normal physiological temperatures, or static cold storage, which chills organs in a preservation solution in an ice box, hypothermic machine perfusion pumps a preservation solution continuously through the organ at temperatures between 1 and 10 degrees Celsius.
Today LifePort Kidney Transporter is routinely used in more than 300 transplant programs in 39 countries, with a performance record of more than 112,000 kidney transplant procedures. In France, LifePort Kidney Transporter has been specified as the HMP standard of care nationwide, from donor to recipient bedside, and is used in the majority of deceased donor kidney transplantation surgeries performed in that country.
Compounding success
Building upon the success of LifePort Kidney Transporter, Organ Recovery Systems researchers began developing a second LifePort system for livers, completing the first commercial-built prototype in 2016. As part of the FDA registration process, LifePort Liver Transporter is now in a phase three pivotal non-inferiority clinical trial comparing HMP to static cold storage. Phase three clinical trials compare two or more competing therapies. While most phase three trials aim to demonstrate the superiority of a new treatment in comparison with a control treatment, non-inferiority phase three trials assess if a more convenient, less toxic, or more affordable intervention is at least as efficacious as an existing standard of care.
“Our primary endpoint for the trial is early allograft dysfunction, based on how the liver functions in the first seven days after transplant,” said James Guarrera, M.D., who directs the liver transplantation program at University Hospital in Newark, New Jersey, and serves as co-lead investigator for the LifePort Liver Transporter clinical trial. “We are following patients out to a year, because a one-year graft survival time point is such an important metric in the U.S.,” he said. With those results, Guarrera says that transplant professionals will be able to “really take a critical look at the technology and then decide if they want to utilize it.”
Guarrera has been researching the potential of HMP for liver transplantation for nearly two decades. As a faculty member at Columbia University in 2010, he was lead author on the first published human prospective trial of liver HMP. The pilot study conducted at Columbia University used a prototyped perfusion system developed by the Columbia research team to reduce early allograft dysfunction in the HMP livers compared to static cold storage livers. Though these were small and non-randomized studies, the researchers demonstrated “a reduction in ischemia reperfusion injury, a reduction in early allograft dysfunction, less biliary complications and a shorter length of stay in the hospital,” Guarrera said. That research helped form the clinical basis for Organ Recovery Systems’ LifePort Liver Transporter.
Continuous improvement
Beyond ease of use, the LifePort Liver Transporter offers a benefit to transplant teams in its portability. Weighing about 100 pounds when fully loaded, it can be handled by two people. Already in the current clinical trial, livers have flown at least five times without issues.
“Functionality, in parallel with making sure that it was as transportable as possible, was really important,” Kravitz said.
United Network for Organ Sharing chief medical officer David Klassen, M.D., expects research to eventually reveal that any form of machine perfusion — normothermic or hypothermic — is superior to the current standard of static cold storage, but no research as of yet has conclusively compared normothermic to hypothermic or established a standard-of-care perfusion protocol. There is a growing body of evidence, however, that machine perfusion can safely extend preservation time. With allocation policies evolving to promote broader geographic sharing, organs are likely to continue traveling longer over greater distances. Particularly for the most time-sensitive organs, such as livers, “this technology has big implications for allocation policy,” Klassen said.
It is in the longer distances and travel times made possible by HMP that Guarrera believes LifePort Liver Transporter offers a potential benefit. Additionally, in the event of technical failure, LifePort Liver Transporter is designed to fail safely, and still function as an effective static cold storage device. “You have the safety of that gold standard backup of cold storage,” Guarrera said. “As we are potentially sending livers over longer distances or don’t have experienced teams going with the organ, that is going to be a huge plus.”
For Kravitz, the experience of his own father’s transplant continues to inspire Organ Recovery Systems’ mission, which is focused on improving outcomes, lowering the cost of transplantation and honoring the gift of life. “It’s a passion for us, not a job,” he said.
Disclaimer: Any reference obtained from this article/publishing to a specific product, process, or service does not constitute or imply an endorsement by UNOS of the product, process, or service, or its producer or provider. The views and opinions expressed in any referenced do not necessarily state or reflect those of UNOS.
Organ: Kidney, liver
FDA status: 510(k) for LifePort Kidney Transporter, IDE for LifePort Liver Transporter
Current clinical trial: U.S.: Study to Evaluate Performance of LifePort® Liver Transporter System, a Machine Perfusion System, for Liver
The post appeared first on UNOS.
Audience:
Heart transplant program directors, surgeons, physicians, administrators, clinical coordinators and data coordinators; compliance and quality managers; clinical support staff; OPO executive directors and procurement directors/managers
Implementation date:
January 9, 2020
Overview of Changes
On Thursday, Jan. 9, several OPTN policy updates will be implemented to eliminate the use of donation service areas (DSAs) from heart transplantation and replace them with a 250 nautical mile circle. Additionally, the term “zone” will be removed from thoracic (heart and lung) allocation policies and replaced with the actual distances in nautical miles between donor hospital and transplant hospitals where candidates are listed. (Previous policy action in 2017 removed DSA from lung allocation). An additional changes will remove current policy language regarding prioritization of sensitized candidates within a DSA.
All of the policy updates are included in this policy notice, reflecting actions the OPTN Board of Directors approved at its June, 2019 meeting.
System Implementation Information
To implement these changes, the UNOS Secure EnterpriseSM system, which includes UNetSM and other transplant applications such as WaitlistSM, DonorNet®, TIEDI® and KPDSM, will be unavailable on Thursday, Jan. 9, from 7:00 a.m. until approximately 7:30 a.m. EST.
The policy changes do not affect definitions of “local” thoracic candidates used in:
- donor acceptance criteria within Waitlist
- contact management within DonorNet
- notification limits for organ offers
Review processes described in the OPTN Member Evaluation Plan are not affected by any of these updates.
Resource information
- A resource document, “Thoracic Allocation Using Nautical Miles,” is available in UNOS Connect from the Heart category of the course catalog, offering HRT105-D
- Online help documentation covering UNetSM functionality will be available when the system updates go into effect. Access Secure Enterprise and then choose Waitlist. On the Help menu, click Waitlist Help. You may search for a specific help topic or use the table of contents to assist with your search.
Questions?
If you have questions about the implemented updates, contact UNOS Customer Service at 800-978-4334. For policy-related questions, send an e-mail to member.questions@unos.org or call 844-395-4428.
The post appeared first on UNOS.
Meet the OC team and learn how they work hand in hand with OPOs and transplant centers to place organs every day, around the clock.

The Organ Center at UNOS has been open for 35 years, around the clock, helping organ placement and transplant centers to deliver the gift of life.
In this series of videos, you’ll hear from Organ Center Director Roger Brown as he talks about how his staff works with the donation and transplant community to meet the daily challenge of matching organs and saving lives. Plus, how OC staff are trained, what motivates them, and the story of a 1-in-1,000 chance connection that gave a struggling firefighter a long-awaited chance for a longer life.
In continuous operation for more than 35 years, the F.M. Kirby Foundation Organ Center has been described as the “heart” of UNOS. Like a beating heart, it’s at work continuously—days, nights, weekends, holidays and during inclement weather and even national disasters—saving lives by matching donors with candidates on the transplant waiting list.
What does the Organ Center do?
- Assist in placing donated organs for transplantation
- Assist in gathering donor information and running the donor/recipient computer matching process
- Assist with transportation of organs and tissues for the purposes of transplantation
- Act as a resource to the transplant community regarding organ-sharing policies
Read more about the Organ Center’s mission and history.
Audience
All DonorNet® match users
On July 24, 2019, we added a new report feature to the DonorNet match page that shows the distance in nautical miles from the donor hospital to each transplant hospital that appears on the match run. To access this feature, users can simply click on the organ link titled “Show distance to transplant center(s)” which appears at the top of the match run page.
This feature is currently visible for heart, lung and heart/lung matches because only those organ allocation policies classify candidates based on distance in nautical miles. We will expand the availability of this feature when we implement OPTN policy changes for other organs that use distance to classify candidates.
Contact us
If you have questions, please contact UNOS customer service at unethelpdesk@unos.org or (800) 978-4334.
The post appeared first on UNOS.
Richmond, Va. – The OPTN Board of Directors, at its meeting June 10, approved new geographic areas to match heart and vascularized composite allograft (VCA) transplant candidates with available organs from deceased donors. Both systems will now be based on distance from the donor hospital to the transplant hospital, replacing fixed and irregular donation service area (DSA) and regional boundaries.
“We continue to refine the organ distribution process to create the most equitable access for all people in need of a transplant,” said Sue Dunn, president of the OPTN Board. “The new distribution systems will help us do so, and they have been well supported by clinicians and the public. They also are in keeping with federal regulatory guidance to minimize differences in transplant access based on where candidates live or which transplant hospital they choose.”
Under the revised policy for heart and lung allocation, the most local level of heart distribution will be for transplant candidates listed at hospitals located within 250 nautical miles of the donor hospital. This replaces all references to the hospitals within the DSA where the heart is recovered. Lung distribution policy was updated in November 2017 to replace local DSA boundaries with the same distribution area (transplant candidates listed at programs within 250 nautical miles of the donor hospital).
VCA distribution will now begin with compatible candidates listed at transplant hospitals within a 500 nautical mile distance from the donor hospital. The great majority of VCA transplants performed up until now have come from donors within this distance.
Other key actions
In other action, the OPTN Board approved an amendment to the OPTN’s variance for the HIV Organ Policy Equity (HOPE) Act. Programs meeting HOPE Act research and experience requirements will now be able to recover additional organ types from donors identified as HIV-positive and transplant them into candidates who are also HIV-positive. This measure broadens organs transplantable from liver and kidney to also include heart, lung, pancreas, kidney-pancreas and intestinal organs.
The Board amended a proposed voluntary national protocol encouraging the greater use of split liver transplants (dividing a liver from a deceased donor into two segments that may be transplanted into two different candidates). After discussion, the Board approved the protocol for use in OPTN Region 8, which had requested it on a regional basis prior to the proposed national variance. Other regions may apply later for a similar protocol if they choose.
The Board also endorsed two guidance documents: one outlining ethical implications of multi-organ transplants and one describing effective practices in broader organ distribution.
About the OPTN Board of Directors
The Organ Procurement and Transplantation Network (OPTN) Board of Directors establishes and updates OPTN policies and bylaws, consistent with statutory and regulatory requirements. The Board consists of 42 volunteer experts from around the country in organ procurement and transplantation as well as organ recipients, living donors, and donor families. The Board is supported by the efforts and recommendations of 21 OPTN committees, also comprised of volunteers representing the diversity of people, perspectives and expertise involved in the nation’s organ transplant network.
United Network for Organ Sharing (UNOS) serves as the OPTN under federal contract.
The post appeared first on UNOS.
Learnings from the pilot will help inform a national rollout
The first phase of the UNOS imaging study pilot concluded in April. During the pilot, the six participating OPOs uploaded 1,153 image studies from 385 donors. These studies were viewed 4,101 times by 763 individual users. UNOS is using the pilot to solidify plans to rollout a large-scale, national donor image sharing platform in DonorNet®. What did they learn?
- Pilot usage data indicates that most OPOs will upload images for all (100 percent of) heart donors and 85 percent of non-heart donors with other organs recovered.
- Based on historical data, a national system would need to support around 5,000 donor image uploads a month.
Clinical and administrative staff from six OPOs and many transplant hospitals contributed to the pilot. They provided useful feedback through conversations, surveys and a UNOS innovation event with surgeons. Among their suggestions for improvement:
- Better mobile access
- A way to securely and easily share imaging studies with non-UNetSM users
- Allow OPOs to forward studies to image interpretation services
- Allow donor hospital to send image studies directly to DonorNet
With continued help from donation and transplant professionals, the UNOS team will build system enhancements into the national plan. We will provide additional information as plans progress.
The post appeared first on UNOS.

Using the new Center Acceptance and Refusal Evaluation (CARE) tool, transplant centers can see all of the outcomes for organ offers they accept as well as all of those they refuse.
This interactive tool allows transplant centers to review their own organ acceptance rates for specific types of donors, along with transplant specific and aggregate outcomes information on the organs they refused that were transplanted elsewhere.
“The CARE tool offers instantaneous visualization of transplant centers’ organ utilization,” says Scott LeHew, Business Development Manager, UNOS Solutions, “and a better understanding of the decisions they’re making regarding organ refusal and acceptance.”
Updated monthly, the tool includes information on all organs (other than VCA) offered to the center during the most recent two-year period available. The data are divided into two separate reports by organ type—thoracic, which includes data on offers to heart, lung, and heart-lung patients, and abdominal, which includes data on offers to kidney, pancreas, kidney-pancreas, liver, and intestine patients.
The report features at least three separate visualizations for each organ type: Accept, Offers, and Survival.
For kidney and kidney-pancreas offers, a fourth visualization is provided to review the rate of delayed graft function of organs accepted by the center as compared to those refused and transplanted elsewhere.
ACCEPT
The Accept visualization allows the user to view the number and percentage of organs (donors for kidneys, lungs, and split livers) offered and accepted by the center during the two-year period. Only those offers where the center had the opportunity to accept the organ (i.e. the center had at least one “primary” offer and their first candidate was above the last acceptor on the match run) are included in the calculation of these rates.
These data can be filtered by a variety of different match, donor, and organ characteristics, including Recovery OPO and Distance to Donor Hospital.
OFFERS
The Offers visualization provides the user with a detailed listing of all the offers that were displayed in aggregate on the Accept page.
These data can be filtered by a variety of different match, donor, and organ characteristics similar to Accept reports with some additional filters, including Primary Refusal Code if users only want to review refusals for Donor Age/Quality, or Patient ID/Unavailable.
SURVIVAL
The Survival visualization provides aggregate Kaplan-Meier graft and patient survival rates at six months and one year post-transplant. It compares organs the center transplanted with those the center was able to accept for at least one candidate and were ultimately transplanted at another U.S. transplant center.
Filters for this visualization are specific to the applicable donor characteristics of the organ type analyzed. For all organ types, filters include Declined for Donor Related Reasons and Survival Types, which allow the user to toggle between patient and graft survival rates.
DGF
This visualization is only available for kidney and kidney-pancreas transplant. Similar to the Survival visualization, this piece of the tool allows the user to compare the Delayed Graft Function Rate (defined as need for dialysis within first week post transplant, collected on Transplant Recipient Registration form) for those organs they transplanted with the organs they refused for at least one candidate at their center and were ultimately transplanted at another U.S. center. The filters available are similar to those in the Accept and Offer visualizations for the same organ types.
Learn more about custom data solutions offered by UNOS.
In focus
OPOs set records for organ donation in 2018
71 percent of organ procurement organizations increased organ donations in 2018, thanks to the generosity of donors and donor families.
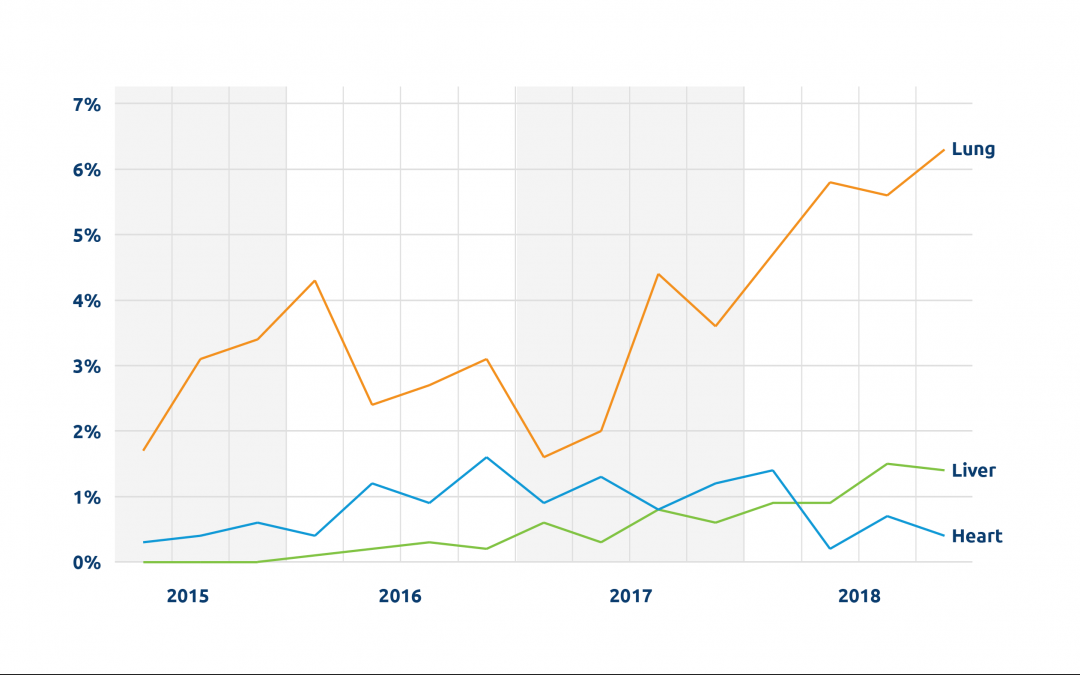
Lung and liver perfusion on the rise
Lung perfusion has more than tripled since 2015, growing from 1.7 percent to 6.3 percent and potentially expanding the pool of organs available for transplant.
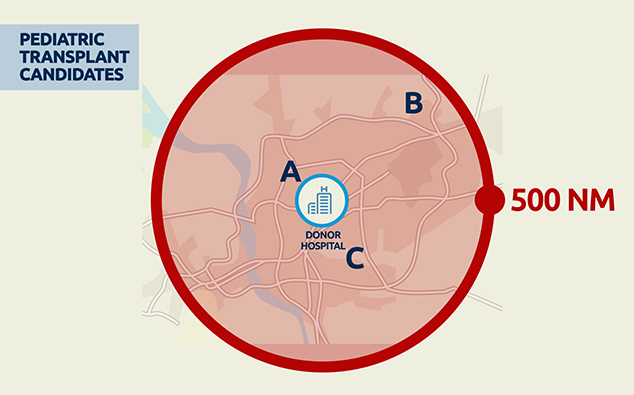
How will the new liver system help pediatric patients?
More pediatric patients will receive transplants as a result of new OPTN liver distribution policy
The post appeared first on UNOS.

INSIGHTS
Game changers
Companies at the forefront of organ perfusion technology
“We work on the lungs like a team works on a patient.”
Martine Rothblatt, PhD, Chairman & CEO, United Therapeutics
Company headquarters: Silver Spring, Maryland
Organ: Lung
Current clinical trials: FDA approval received for Phase 3 trial for 16 centers and 93, or fewer, EVLP transplants.
One significant challenge to widespread implementation of NMP is having the technology and skilled clinical specialists available when needed. Lung Bioengineering, a United Therapeutics subsidiary, seeks to address this challenge with freestanding, centralized perfusion facilities staffed around the clock, to serve multiple transplant centers. Initially unacceptable/marginal lungs are transported to the facility and perfused.
Real-time HD monitoring allows the transplant team to examine the lung remotely during perfusion; if deemed acceptable, the lung is returned to the transplant center. “We work on the lungs like a team works on a patient,” notes United Therapeutics’ Chairman and CEO Martine Rothblatt, PhD. She says during the Phase 2 clinical trial, about two-thirds of the initially unacceptable lungs received by the center were recovered, accepted and successfully transplanted, with perfusion performed on the Toronto EVLP System technology developed at University Health Network in Toronto, Canada. (Lung perfusion services are also available for transplant centers not participating in the trial, with the XVIVO XPS device under the FDA’s HDE approval.)
“Ultimately our goal is to ramp up the availability of lungs for transplant,” says Rothblatt; the company hopes to establish four perfusion centers nationwide with the goal of doubling the number of lung transplants performed annually in the US.

An EVLP Suite

Lung Bioengineering’s headquarters in Silver Spring, Maryland
“Ideally speaking, every organ that is salvageable that has the potential to be transplanted should be assessed and should be allowed time on perfusion technology without impact of ischemia and cold static preservation.”
Tamer Khayal, M.D., Chief Commercial Officer, TransMedics
Company headquarters: Andover, Massachusetts
Organ: Lung, heart, liver
FDA status: PMA for OCS lung, IDE – EA for OCS heart, IDE for OCS liver
Current clinical trials:
- U.S. OCS Heart EXPAND
- U.S. The Organ Care System (OCS
 ) Lung Thoracic Organ Perfusion (TOP) Post Approval Study (PAS) Registry – OCS
) Lung Thoracic Organ Perfusion (TOP) Post Approval Study (PAS) Registry – OCS Lung TOP PAS Registry
Lung TOP PAS Registry - International OCS Lung EXPAND
- International OCS Liver PROTECT
The OCS Lung is currently “the only PMA approved medical technology for ex-vivo perfusion and assessment for routine donor/standard criteria lung transplants in the US,” says TransMedics Chief Commercial Officer Tamer Khayal, M.D. The OCS—which the company has developed for lung, liver, and heart transplantations—is a portable device for NMP that makes it possible to perform perfusion continuously from donor recovery to recipient.
OCS devices were designed to minimize ischemic injury, in comparison to static cold storage preservation. The OCS also allows for diagnostic monitoring of organ viability or function and therapeutic or optimization capabilities during perfusion.
The TransMedics vision is that “ideally speaking, every organ that is salvageable that has the potential to be transplanted should be assessed and should be allowed time on perfusion technology without impact of ischemia and cold static preservation,” says Khayal.
To date, OCS devices have been used in nearly 1,200 transplants worldwide.
“Risk and urgency are the two areas we are tackling head-on with this technology.”
Craig Marshall, CEO, OrganOx
Company headquarters: Oxford, England
Organ: Liver
FDA status: Clinical trial IDE
Current clinical trials: U.S. OrganOx metra®
“Risk and urgency are the two areas we are tackling head-on with this technology,” says OrganOx CEO Craig Marshall.
The metra® (pronounced meetra, from the Greek for womb) is a transportable normothermic machine perfusion (NMP) device that is now in a multi-center Investigational device exemption (IDE) clinical trial in the U.S. comparing the safety and efficacy of preservation and transport on the device to conventional static cold storage (SCS).
“By making it possible to assess the liver ex vivo, we are attempting to reduce the risk associated with lack of information about an organ,” says Marshall. And with clinical data from Europe indicating that livers can be perfused safely for up to 24 hours on the metra®, the device, Marshall says, benefits transplant surgery teams and recipients alike by reducing the urgency of the critical time limits imposed by SCS.
Surgeons David Nasralla and Annemarie Weissenbacher inspecting a liver perfusing on the OrganOx metra® device
“We can offer an additional tool in the clinician’s toolbox to assess lungs that might previously have not been utilized.”
Dan Martinelli, Head of Sales America, XVIVO Perfusion
Company headquarters: Gothenburg, Sweden
Organ: Lung
FDA status: HDE “for flushing and temporary continuous normothermic perfusion of initially unacceptable excised donor lungs.”
Current clinical trials: None
Directed by the company’s vision “that nobody should have to die waiting for an organ,” XVIVO focuses on expanding the use of ex vivo lung perfusion to increase the number of transplantable lungs, says XVIVO Head of Sales Dan Martinelli.
In the completed NOVEL clinical trial, initially unacceptable extended criteria/marginal donor lungs were assessed for transplant via NMP on the XPS device.
Before perfusion, “the majority of those lungs were turned down by the majority of the transplant centers” enrolled in the trial, says Martinelli. Those found acceptable following perfusion and active evaluation, however, were successfully transplanted and demonstrated outcomes—including three-year survival rates—comparable to standard criteria lungs.
“It is the first trial in the U.S. that specifically looked at marginal lungs” and resulted in the FDA’s HDE approval for the XPS, notes XVIVO clinical research program manager Jaya Tiwari. Now, says Martinelli, the XPS can offer “an additional tool in the clinician’s toolbox” to assess lungs that might previously have not been utilized.
Photos courtesy of XVIVO Perfusion
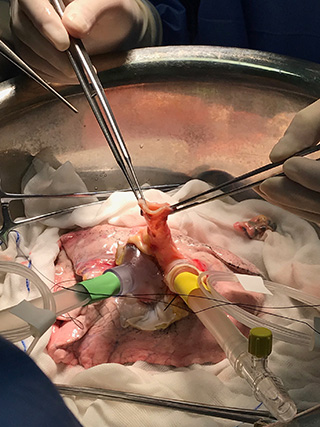
Learn more
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world.
FDA Terms
The post appeared first on UNOS.
Chair of the OPTN Transplant Administrators Committee previews opening session at TMF
“The transplant administrator, at the end of the day, is really the heart and the mind of the transplant program. They keep it running. They make sure their center is appropriately staffed, that they recruit the right team, and that they have a positive work environment. It’s a demanding job because it requires a kind of maestro to orchestrate all of that and keep everyone in sync. It’s non-stop, 24/7.”
It’s a job James Pittman, RN, MSN, knows well. The current chair of the OPTN Transplant Administrators Committee began his work in transplant administration at the former Methodist Hospital in Houston (now Houston Methodist at Texas Medical Center). Today, he serves as Assistant Vice President of Transplant Services for Nashville-based HCA Healthcare, which comprises 185 hospitals in the U.S., including 13 transplant centers across the country.
“But I’ve always been in transplant, since the beginning when I was at the bedside.” After graduating from nursing school at the University of Texas at Austin, he started out in the ICU, where he cared for heart and lung transplant recipients.
Pittman says among the many issues transplant administrators must navigate, are new allocation policies: “It’s a big topic right now, and we have to be accountable to all of that. And we have to be the ones who have the vision and foresight to mitigate any sort of risk we might encounter and do it proactively and find solutions.”
When he gives remarks at the TMF opening welcome session this year, Pittman will be speaking to a broad community of thought leaders in the field at what he describes as the preeminent education event for transplant administration.
“When physician leaders and hospital executives and up-and-coming future administrators attend TMF, it’s of great benefit to the entire community. To really focus on the art and practice of administering and leading a transplant program, we as administrators need partners in all of our aspects.”
Connect with James Pittman and other transplant administration leaders at the 27th Annual UNOS Transplant Management Forum in Orlando, May 13-16, 2019.
More about the OPTN Transplant Administrators Committee (TAC)
TAC considers issues related to the administration of transplant programs and provides input to the other Committees and the Board with regard to the potential impact of developing policies and other OPTN requirements on transplant program operations. Through Non-OPTN resources provided by UNOS as available, the committee develops initiatives and tools that foster effective transplant program administration such as the annual UNOS Transplant Management Forum, the transplant program staffing survey, and the standardized payer Request for Information (RFI) tool. Find out more about the committee here.
The post appeared first on UNOS.

Insights: FEATURE
A look at the rapidly evolving technology that could make more organs available than ever before.
Across five decades of advances in transplant medicine, static cold storage (SCS) has remained a largely unchanged but vital link in the chain between donor and recipient. Yet static cold storage has well-recognized limitations. SCS slows, but doesn’t prevent, ischemic injury, which in turn increases the risk of graft non-function, ischemia reperfusion injury, primary graft dysfunction, and other post-transplant complications in the recipient. In addition, only a limited assessment of the organ is possible during SCS, making it difficult to accurately predict how well an organ may function following transplant.
Now, however, the rapidly advancing technology of ex vivo normothermic machine perfusion (NMP) holds promise for improved preservation, better assessment and even reconditioning of organs before transplant. And while many questions remain to be resolved regarding the implementation of this still-young technology, already NMP has had an important impact on transplant medicine in the U.S. (and globally), expanding the donor pool by allowing surgeons to assess and successfully transplant organs that once would have gone unutilized.

In comparing NMP with SCS, a primary benefit the technology appears to offer is the luxury of time …
Preserving time
The basic principle of NMP technologies is to approximate “near physiologic” conditions of temperature, nutrients and oxygen outside the body and to enable the organ to function much as it would within the body. Videos of ex vivo NMP vividly illustrate the concept—a heart beats, lungs expand.
In comparing NMP with SCS, a primary benefit the technology appears to offer is the luxury of time; although a safe time limit has not yet been established for NMP, clinical evidence suggests that organs are substantially protected from ischemic injury while being perfused—in essence, NMP “stops the clock” on ischemic injury. “Extending preservation time is a clear benefit, and that is solid and well-established,” says UNOS Chief Medical Officer David Klassen, M.D., former director of the kidney and pancreas transplant programs at University of Maryland Medical Center.
NMP may make it possible, then, for organs to be transported over greater distances, for recipients to travel further to a transplant center, and for surgeons to have more flexibility to schedule optimal surgical times and increase surgical time for complex recipient cases.
![]()
In essence, normothermic machine perfusion (NMP) “stops the clock” on ischemic injury.
Developing the technology
Three companies are currently leading the development of ex vivo NMP in the U.S., with devices in current and/or recently completed clinical trials.
Massachusetts-based TransMedics offers the transportable Organ Care System (OCS) devices for heart, lung, and liver; OrganOx, based in the UK, has developed the metra® transportable perfusion device for liver; and Swedish-headquartered XVIVO Perfusion offers the non-transportable XPS lung perfusion system.
A fourth company, Lung Bioengineering, based in Maryland, has focused on making perfusion technology more widely accessible by developing a centralized, free-standing perfusion facility to serve multiple transplant centers. The facility employs both the XPS and the Toronto EVLP System developed at University Health Network in Toronto, Canada.
Assessing function and expanding the donor pool
Perhaps most important among its many potential benefits, NMP makes it possible for organs to be functionally evaluated. When surgeons have had to make educated judgements about the condition of donor organs, potentially usable organs—particularly those considered “marginal” or “extended criteria”—have inevitably gone unutilized. This problem is a particularly critical factor in the low utilization rate for donor lungs; only about 20-25 percent of lungs are transplanted from all deceased donors, according to UNOS research scientist Rebecca Lehman, PhD, and more than half are never even recovered for the purpose of transplant.
But with NMP, says Duke University lung transplant surgeon Dr. Matthew Hartwig, “The devices allow us in a very safe and reproducible fashion to assess the lung outside of the complex environment of the donor and to evaluate the function of the lung in isolation.” Using NMP through clinical trials at Duke, says Hartwig, has made it possible to more objectively determine which organs are most likely to be successfully transplanted. As a result, “We have been able to utilize some lungs that otherwise would not have been used for transplant but that could have been and should have been,” he says.
One such trial was Lung Bioengineering’s multicenter Phase 2 clinical trial, which ended in August of 2018. Initially unacceptable lungs were transported to the company’s Silver Spring perfusion center to be assessed; all lungs that were deemed acceptable after being evaluated via NMP were successfully transplanted.
“If they do well in ex vivo, they do well in transplant,” confirms transplant surgeon Dr. Pablo Sanchez, director of the ex vivo lung perfusion program at the University of Pittsburgh, another of the Lung Bioengineering clinical trial locations.
OrganOX metra®, a transportable liver NMP device now in clinical trials in the U.S.
Comparable benefits have been seen for other organs. In a UK trial of the OrganOX metra®, a transportable liver NMP device now in clinical trials in the U.S., 31 livers initially declined by all seven UK liver transplant centers were assessed on the device, and 22 were successfully transplanted, according to Craig Marshall, CEO of OrganOX.
As Marshall notes, there is “risk associated with lack of information about an organ”—being able to “assess an organ ex vivo and make an informed decision” increases information and decreases that risk. Clinical data thus far seem to indicate comparable outcomes for initially unacceptable or extended-criteria organs transplanted following assessment via NMP—offering the promise that many more organs, such as those from DCD donors, will prove safe for transplant to help address critical shortages.
Video courtesy of XVIVO Perfusion

Lung Bioengineering’s EVLP Suite
Up close
Leading the Way
Meet the companies developing ex vivo NMP in the U.S.
TransMedics, Andover, Massachusetts, Transportable Organ Care System (OCS ) devices for heart, lung, and liver
) devices for heart, lung, and liver
OrganOX, Oxford, England, metra® transportable perfusion device for liver
XVIVO Perfusion, Gothenburg, Sweden, Non-transportable XPS lung perfusion system
Lung Bioengineering, Silver Spring, Maryland, Centralized, free-standing perfusion facility serving multiple transplant centers
Future potential, future questions
Beyond the immediate benefits already being demonstrated, NMP technology is widely believed to have the potential to powerfully transform the future of transplant medicine.
Although clinical trials have focused on demonstrating the safety of using ex vivo NMP before transplant, researchers and transplant professionals believe that the same technology will likely soon make it possible to improve the condition of recovered organs or even treat them therapeutically for problems such as bacterial or fungal infections, with the goals of further expanding the donor pool and also improving post-transplant outcomes for recipients.
And in the long term, biotechnology experts hope organs could be modified through ex vivo perfusion to prevent or reduce the need for anti-rejection medications, a development that could support advances in xenotransplantation—the modification of nonhuman organs to use in human transplant.
For now, however, basic questions still need to be addressed in the implementation of this technology:
- What criteria should determine whether an organ is perfused, or should NMP become standard protocol for all donor organs?
- Where, when, and for how long should NMP be applied?
- Will the possible benefits of NMP outweigh the costs (including equipment, training, and personnel) of implementing this technology, and how will those costs be distributed across the OPTN?
“We still have a lot of work to do as a community to determine what is going to be the best way to perfuse, evaluate, rehabilitate and eventually improve the organ in an ex vivo setting,” says transplant surgeon Matthew Hartwig. Nevertheless, he believes the day will come, he says, when “the idea of cold storage will be a historical footmark that people will chuckle about as the way we used to do transplant.”
The post appeared first on UNOS.
The new French allocation system based on the candidate risk score (CRS) considered donor-recipient matching and implemented nationwide donor heart sharing. In candidates for whom the CRS did not predict accurately waitlist mortality score exceptions can be requested. This study aimed to describe the heart allocation modalities and donor-recipient matching since implementation of the new system on January 2, 2018.
Improved utilization of donor hearts would benefit chronic heart failure patients awaiting transplant. Limitations to cardiac allograft preservation include ischemic time, donor organ quality, and accurate graft assessment. We hypothesized that human hearts declined for transplant may be resuscitated after varied ischemic times. The unique studies performed within the Visible Heart® Laboratory for over two decades have provided unique insights relative to optimizing large mammalian functions post-cardioplegia.
Ex situ heart perfusion (ESHP) is a novel method for preservation of the donated heart in a semi-physiologic state and provides the opportunity to evaluate pre-transplant function. Because of the well-described extraordinary metabolic requirements of the heart, efficient metabolic support is critical for optimal preservation of function and viability. Our aim was to determine changes in energy metabolism/energy substrates during extended ex situ perfusion of hearts in two different perfusion modes, working mode (WM) and non-working mode (NWM).
The OCS Heart EXPAND Trial is a prospective, multi-center trial to evaluate the effectiveness of the OCS™ to resuscitate, preserve and assess donor hearts that may not meet current standard donor heart criteria for transplantation to potentially improve donor heart utilization for transplantation.
At present the assessment of the heart transplant (HTx) recipient’s personal alloimmune risk is still not precise enough. The aim of this study was to evaluate the impact of molecular-level human leukocyte antigen (HLA) matching on post-transplant graft survival, rejection and cardiac allograft vasculopathy (CAV).
To describe technical aspects of angiography and intravascular ultrasound (IVUS) in ex-vivo perfused hearts using the Organ Care System (OCS, TransMedics, Andover, MA USA).
The new French allocation system (NFAS) based on the candidate risk score has been launched on January 2, 2018. This study aimed to evaluate the effect of implementation of NFAS on newly registered candidates’ profile and their waitlist outcomes.
The lack of donor organs limits the availability of heart transplantation as an optimal treatment option for advanced heart failure patients. In an effort to expand a pool of acceptable donor organs we explored a correlation between donor age and 1-year outcome in patients undergoing heart transplantation.
Risk stratification and early detection of cardiac allograft vasculopathy (CAV) are essential in heart transplantation patients. CAV presents a diffuse vascular involvement, and has been difficult to noninvasively diagnose by the lack of a sensitive method to detect developing vascular pathology in the allograft. The present study investigates the ability of 13N-ammonia PET for detection of CAV in heart transplant patients.
Primary graft dysfunction (PGD) is a leading cause of mortality and morbidity following heart transplantation. Our study aimed to determine the feasibility of measuring cardiac biomarkers in the donor heart directly after preservation and use as predictors for PGD.
In the United States, heart allocation is administered by the United Network for Organ Sharing (UNOS). UNOS collects demographics and clinical data for all transplant candidates, donors, and recipients listed since 1995. While the data is available to the public upon requests; it has been used predominately for research rather than clinical decision making, transplant patient use and policy makers. The aim of this project is to build a web application to visualizes UNOS data and promote easy access to summary statistics for diverse members of the transplant community.
Among patients who undergo orthotopic heart transplant (OHT), intravascular ultrasound (IVUS) detection of coronary allograft vasculopathy (CAV), defined as ≥ 0.5 mm increase in maximal intimal thickness (Δ MIT), is associated with worse outcomes at 5 years. Other IVUS-derived measurements of arterial plaque, while validated in studies of coronary atherosclerosis, have not been well studied in CAV.
Despite the controversial effect of elevated heart rate on progression of cardiac allograft vasculopathy (CAV), heart-rate-slowing agents are frequently prescribed with the assumption that higher heart rate predicts worse outcomes in cardiovascular disease.
Psychosocial assessment is a key component of heart transplantation (HT) evaluation. Criteria such as patient compliance to medical therapy, level of social support, and history of substance abuse are important considerations when assessing appropriateness for listing. The aim of this study was to characterize the patients who do not satisfy listing criteria due to psychosocial criteria and assess the reasons for which they are rejected.
Our hypothesis is that by improving access to advanced heart failure specialists (AHFS) through partnered outreach clinics, we can increase the number of patients benefitted by advanced heart failure (AHF) therapies delivered at our center. This partnered network approach can potentially overcome distance barriers, optimize referral timing, and expedite access to AHFS from the regional center of excellence.
With the growing shortage of organ donors, marginal donor organs are increasingly accepted, which may partially explain the continued increase in donor age. The potential interactions between donor-recipient (D/R) age difference and outcomes after heart transplantation (HT) are not well known, and organ allocation systems do not routinely consider D/R age matching. We thus aimed to study the impact of D/R age difference on HT outcomes.
In this issue of the Journal, Profita and colleagues report findings from a well-controlled analysis matching outcomes from the Organ Procurement and Transplantation Network (OPTN) and the Extracorporeal Life Support Organization (ELSO) datasets to identify incidences of and risk factors for severe primary graft dysfunction (PGD) in pediatric heart transplant recipients within the United States.1 The authors find no change in the incidence of severe PGD across two decades of pediatric heart transplant.
We previously reported a microarray-based diagnostic system for heart transplant endomyocardial biopsies (EMBs), using either 3-archetype (3AA) or 4-archetype (4AA) unsupervised algorithms to estimate rejection. The present study aimed to examine the stability of machine-learning algorithms in new biopsies, compare 3AA vs. 4AA algorithms, assess supervised binary classifiers trained on histologic or molecular diagnoses, create a report combining many scores into an ensemble of estimates, and examine possible automated sign-outs.
Pilot project tests new system that could offer quick access to high-quality medical images and facilitate more transplants
By Judy Ivey

UNOS business architect Rob Mctier (standing) working with the technology development team.
A medical image sharing pilot project underway now at UNOS may soon give organ procurement organizations, and donor and transplant hospitals universal access to high-quality medical imaging studies during the organ offer process. Creating a consistent, reliable and secure national image sharing system has the potential to decrease the number of organs that are not used and increase the number of transplants overall.
How image sharing often happens now
When the procurement team at Baltimore-based Living Legacy Foundation of Maryland needs images of a donor’s organs, they often take them with their iPhones. The OPO has more than 150 donors each year.
The images are emailed or sent via text to Living Legacy’s communications center and then shared with surgeons who request them explains Debbi McRann, Chief Clinical Officer. “But the quality isn’t good and we can’t upload it to DonorNet® because the file is too big. We’re only giving surgeons a partial scan because of the limitations.”
DonorNet® currently accommodates certain images as attachments, but file sizes are limited and the process of acquiring and attaching images is inefficient, preventing routine use.
When surgeons can’t clearly see the organ being offered, given the risk of travel to recover and the possibility that the organ won’t meet their patient’s needs when they see it in person, it stands to reason that they would hesitate to accept some offers. This results in unused organs that could otherwise help save patients’ lives.
“Another one of the challenges we’ve been having when we receive offers from outside centers is that they will have multiple donors listed, so the risk is it’s not linked to the actual donor record so you could potentially download the wrong CT and send it to the surgeon,” explains McRann.
The pilot includes functionality that addresses this risk. When an OPO uploads an imaging study, DonorNet® compares the patient name, gender, and date of birth on the imaging study with the donor name, gender, and date of birth in DonorNet®. If there are differences, DonorNet® warns the OPO user and allows the OPO user to resolve the conflicting data before displaying the imaging study to transplant hospital users.
While some OPOs use image sharing systems, the existing process can be costly, involving multiple vendors and external applications that don’t necessarily talk with each other.
The pilot project currently underway could make a high quality universal donor image system a reality.
UNOS is partnering with New York City-based image sharing provider Ambra Health and six OPOs around the country to test a solution that would make available large file-size medical images, radiographs, videos of echocardiograms, catheterizations, pulmonary bronchoscopies, and other images to OPOs, and transplant and donor hospitals.
“If we can have a consistent way for everyone to transfer information, that’s going to decrease errors,” says McRann. “Right now, there are many different ways of showing these images. If there were only one, there’d be more standardization and fewer errors” and surgeons could decide to transplant organs they may not have considered otherwise.
Target solution
“This pilot is just the first step,” says UNOS business architect Rob McTier who helms the seven-member team that has spent the last several years developing the system. “We want to be able to provide an infrastructure that will enable the sharing of this information.”
“Donor imaging helps other programs learn from each other,” says Jared Sierkierka, Clinical Operations Manager for Donor Network West, based in San Ramon, California, which adopted an image sharing system in 2011. “Transplant hospitals can look at the causes of hesitation when an organ is declined. Other organizations will look at why others accepted an organ they declined and learn.”
How it works
During the pilot, OPOs receive high-quality imaging studies on CDs or thumb-drives. They login to DonorNet® with their existing username and password to upload the imaging study similar to the way they upload smaller attachments. DonorNet® links the imaging studies to a specific donor’s record in DonorNet®. Transplant hospitals login to DonorNet® with their existing username and password to view the imaging study using a DICOM viewing tool.
If the pilot is successful, the national system could work like this:
- The OPO requests an imaging study for a donor from a donor hospital.
- The donor hospital sends the imaging study to UNOS’s imaging hub.
- DonorNet® will automatically connect the imaging study to the appropriate donor record.
- Both the OPO and transplant hospital will log in to DonorNet® and be able to view the imaging study using a DICOM viewing tool.
“I was amazed when I saw the images,” says Dr. Daniel Jacoby, Director of the Comprehensive Heart Failure Program at Yale School of Medicine and founder and director of Yale’s cardiomyopathy program who tested the system. “They look like you’re looking directly at an echo screen. This is a step forward for our patients.”
Says McRann, “I think, because they’re connected right to the donor record, more surgeons and coordinators will be able to look at the images faster and make their decisions faster. This can speed up allocation.”
What’s next
If the pilot is successful, McTier says UNOS plans to make the system available nationwide. Over the next few months, look for updates on UNOS.org about the pilot, including a Q&A with the development team.
Read more about the pilot and for information about available education resources for participating OPOs.
OPOs participating in the pilot
- Center for Organ Recovery and Education, Pittsburgh, PA
- Donor Network West, San Ramon, CA
- Lifesharing: A Donate Life Organization, San Diego, CA
- LifeQuest Organ Recovery Services, Gainesville, FL
- Nevada Donor Network, Las Vegas, NV
- One Legacy, Los Angeles, CA
The post appeared first on UNOS.
Previous reports of primary graft dysfunction (PGD) in pediatric heart transplant (HT) recipients are limited to descriptive series of children who required extracorporeal membrane oxygenation (ECMO) support shortly after HT. In this study we sought to determine the incidence, risk factors, and survival after severe PGD in pediatric HT recipients.
Audience
All UNetSM users who complete OPTN data collection forms
Implementation
January 15, 2019; quarterly updates
At-a-glance
A cross-functional team of UNOS staff and OPTN members is working on a data governance initiative to improve the consistency and quality of OPTN data collection. The first set of data definitions using a new format are published in UNet online Help as of January 15, 2019.
We will provide a list of revised definitions each quarter, to answer member questions about existing data fields and to clarify new requirements.
More details
This effort aims to provide clear, concise data definitions, improve quality of data, and provide transparency into changes. Clarifications are intended to provide guidance for future data entry; you are not required to amend data submitted before the collection date. The process to create revised data definitions includes reviews by multidisciplinary UNOS staff and the Data Advisory Committee.
Summary of definition changes
| Data Element | System | Form | Description |
| Gender | TIEDI® DonorNet® KPDSM |
DDR and LDR DNR Add/Edit Donor |
Intent is to collect biologic and physiologic traits (sex) at birth. |
| Total Cold Ischemic Time | TIEDI® | Liver TRR | Cold ischemic time starts when the organ is cross-clamped and ends when it is first perfused with warm recipient blood (i.e. first clamp removed in situ). Previous to this change, the hepatic artery and portal vein clamps both had to be removed before ischemic time ended. |
| Prior Cardiac Surgery (non-transplant) | TIEDI® | Heart, Lung and Heart-Lung TCR | VAD should be included in the report of previous cardiac surgeries. |
| Time of implant/initiation | WaitlistSM | Adult Heart Status Justification Form | New data collection element released, initial definition established with implementation of heart allocation policy on 10/18/2018. |
| Patient Using Either Oral Medication or Diet for Blood Sugar Control | TIEDI® | Pancreas and Kidney-Pancreas TRR and TRF | Any anti-hyperglycemic medications should be listed in this field, including oral and non-insulin injectables. |
Where to find the info in Help Documentation
Access Secure Enterprise and then choose TIEDI. On the menu, choose Help and click Online Help. Details can be found under Manage Data – Data Definitions and History of Definition Changes. Record Field Descriptions have also been updated to include each data element.
Background
The OPTN’s secure transplant information database contains all national data on the candidate waiting list, organ donation and matching, and transplantation. Organ transplant institutions use the system to match waiting candidates with donated organs. Institutions also rely on the database to manage time-sensitive, life-critical data, before and after their patients’ transplants.
Contact
If you have questions, please contact UNOS Customer Service at (800) 978-4334 or unethelpdesk@unos.org.
The 36,527 organ transplants performed in the United States in 2018 set an annual record for the sixth straight year, according to preliminary data from United Network for Organ Sharing (UNOS), which serves as the national Organ Procurement and Transplantation Network (OPTN) under federal contract. In 2018, the total number of organ transplants exceeded 750,000 performed since 1988, the first full year national transplant data were collected.
The number of transplants, using organs from both deceased and living donors, increased five percent over 2017. Approximately 81 percent (29,680) of the transplants performed in 2018 involved organs from deceased donors. Living donor transplants accounted for the remaining 19 percent (6,849). The number of living donor transplants represented the highest total since 2005 and increased nearly 11 percent over 2017.
“We are incredibly proud and grateful to have facilitated a record number of lifesaving organ transplants in 2018,” said Sue Dunn, president of the OPTN/UNOS Board of Directors. “We never forget that our work is made possible by the selfless donors and their courageous families who make the powerful decision to give the gift of life. We will continue to work tirelessly to maximize that gift on behalf of the nearly 114,000 who await a transplant.
“In 2018, 10,721 people provided one or more organs for transplantation as deceased organ donors. This was a four percent increase over the 2017 total, and it continues an eight-year trend of record-setting donation.
While the number of potential deceased organ donors varies among different areas of the country due to differences in population size and medical characteristics, increases were noted in many areas. Of the 58 organ procurement organizations (OPOs) coordinating deceased organ donation nationwide, 41 (70 percent) experienced an increase in donors from 2017 to 2018, including at least one OPO in each of UNOS’ 11 regions.
“A key to continuing the success of the field is to support efficient decision-making and improve communications among OPOs and transplant centers,” said Brian Shepard, Chief Executive Officer of UNOS. “We are working on a number of innovation projects to increase the efficiency of these key processes.”
As in several previous years, some of the increase in deceased donation is due to increased usage of donors with a broader set of medical criteria than was considered in the past. Nearly 20 percent of donors in 2018 donated after circulatory death as opposed to brain death. Nine percent of deceased donor kidney transplants involved organs with a kidney donor profile index (KDPI) score of 86 or higher, which may function less time compared to low KDPI kidney offers but may also shorten the waiting time for transplant candidates. Other donor characteristics setting all-time records in 2018 included an age of 50 or older and/or being identified as having increased risk for blood-borne disease.
Rejection with severe hemodynamic compromise (RSHC) carries a mortality risk approaching 50%. We aimed to identify current risk factors for RSHC and predictors of graft failure after RSHC.
Identifying patients who are at risk for complications after thoracic transplantation assists in better defining outcomes and promoting discovery of new mechanisms that can be targeted to mitigate these complications. The gold-standard for diagnosing rejection, a major cause of morbidity and mortality after transplantation, is biopsy, which (1) is not risk-free, (2) may not be practical in critically ill patients and (3) may result in false-negative findings.1 Exosomes had been described as far back as in 1981 as membrane fragments from reticulocytes detected in body fluids.
It’s not the size of a man, but the size of his heart that matters.Evander Holyfield, American professional boxer (1962-)
While survival after pediatric heart transplantation (pHT) has improved in the past few decades and mechanical circulatory support is increasingly used to bridge children to transplantation, waiting list deaths remain a stark reality. To increase the donor pool, strategies such as ABO-incompatible transplantation or donation after cardiac death (DCD) have been attempted.1,2 The real question we have to ask is whether the current donor pool offers are appropriately being used. The article by Davies and colleagues in this issue of the journal looked at the rate of declines in donor offers for pediatric recipients and their consequences.
Heart transplantation is the definitive treatment for end-stage heart failure. A shortage of donor hearts forced transplant programs to accept older donors and longer ischemic times. Previous studies have suggested that administration of mesenchymal stem cells (MSCs) or their conditioned medium (CM) protects the heart against ischemia/reperfusion injury (IRI). We hypothesized that the preservation of donor hearts with a CM would protect the graft from IRI after prolonged storage in 15-month-old rats and investigated mRNA changes attributable to CM.
Pediatric heart transplant waitlist mortality remains significant but allograft offer refusals are common and allografts continue to be discarded.
Traditionally, donor-recipient size match is assessed by body weight. We assessed the ability of 5 size match metrics – predicted heart mass (PHM), weight, height, body mass index (BMI) and body surface area (BSA) – to predict 1-year mortality after heart transplant and to assess the effect on size match on donor heart turn-down for size.
Adults with congenital heart disease (ACHD) represent a growing, albeit still small, proportion of heart transplant (HT) recipients (1, 2). These patients frequently present with complex anatomy and physiology, and atypical manifestations of cardiac failure. Despite being younger than other HT candidates, they have higher risk for adverse outcomes early after HT. This relates to prior thoracic operations, longer allograft ischemic time, a high prevalence of sensitization, less frequent use of pre-transplant mechanical circulatory support (1), and underappreciated end-organ dysfunction.
The history of the SynCardia Total Artificial Heart – temporary (TAH-t; SynCardia Systems, LLC, Tuscon, AZ) is a fascinating medical tale of success, failure and perseverance that has highlighted the lives of many physicians, scientists and patients while episodically consuming the attention of the public. Although, perhaps not as dramatic as its first implantation in a human in 1982, a major and significant milestone in the history of this device was the Food and Drug Administration (FDA) approval of the SynCardia TAH-t in October of 2004 for bridge to transplant indication in the United States, culminating decades of scientific investigation and clinical study and making this device available for commercial use.
Eighty five percent of congenital heart disease patients currently survive to adulthood due primarily to advancements in surgical treatments1. For these survivors with Adult Congenital Heart Disease (ACHD), heart failure (HF) remains the leading cause of death2. Thus, the number of ACHD patients requiring heart transplantation (HT) continues to grow, and with this growth has come improvements in outcomes. In 2009, Lamour et al. demonstrated a 1-year survival of 83% for non-Fontan congenital HT and 71% for patients with Fontan palliation3.
Identification of heart transplant rejection currently rely on immunohistologic and immunohistochemistry. We aimed to identify specific sets of microRNAs (miRNAs) to characterize acute cellular (ACR), antibody-mediated (pAMR) and mixed (MR) rejections in monitoring formalinfixed paraffin-embedded (FFPE) endomyocardial biopsies (EMBs) in heart transplant (HTx) patients.
If you are a patient on the national waiting list for a heart, your urgency for a transplant is currently based on three statuses:
- 1A (most urgent)
- 1B (somewhat urgent)
- 2 (least urgent)
As we learn more about heart disease and successful treatment of it, the transplant community determined we needed more specific criteria that reflects a heart patient’s current health and care they are getting. To accommodate these needs, we are making changes to heart policy and the first phase of the new heart allocation policy will take effect on September 18.
Heart candidates can find specific information here about how this change will affect you. You can also download a print version from the website.
The allocation of donor hearts evolves in response to the changing landscape of advanced heart failure therapies and expanded understanding of donor/recipient matching. The novel, heart allocation algorithm is a natural response to the success of mechanical circulatory support (MCS) and further aligns severity of illness with urgency. In the novel, 6-status system, candidates supported with veno-arterial, extracorporeal membrane oxygenation devices (ECMO) and biventricular, extracorporeal ventricular assist devices (VAD) receive Status 1 urgency, while Status 2 urgency comprises candidates with univentricular extracorporeal VADs, percutaneous, endovascular mechanical circulatory support (e.g.
Decision-making when offered a donor heart for transplantation is complex, and supportive data describing outcomes according to acceptance or non-acceptance choices are sparse. Our aim was to analyze donor heart acceptance decisions and associated outcomes at a single center, and after subsequent acceptance elsewhere.
A new resource booklet, “What Every Parent Needs to Know,” is available for parents and caregivers of children and adolescents who need or receive an organ transplant. The OPTN/UNOS Patient Affairs Committee spearheaded the booklet’s development in collaboration with a number of transplant professionals and parents of organ transplant recipients*.
The booklet explains the transplant process from a parent’s viewpoint. It addresses issues before and during a transplant such as financial concerns and explaining deceased donation to a child, as well as guidance on helping children manage life after a transplant. It addresses a number of concepts and terms relating to transplantation and provides references to other helpful resources.
* The Patient Affairs Committee also wishes to thank the following organizations for reviewing the booklet:
- American Liver Foundation
- American Society of Transplantation
- Anne & Robert H. Lurie Children’s Hospital of Chicago
- Children’s Cardiomyopathy Foundation
- Children’s Organ Transplant Association
- International Pediatric Transplant Association
- National Kidney Foundation
- Pediatric Heart Transplant Study
Richmond, Va. — Members of the national organ donation and transplantation community have elected 25 members to the OPTN/UNOS board of directors, including a new president, vice-president/president-elect, vice president for patient and donor affairs and secretary. United Network for Organ Sharing (UNOS) serves as the Organ Procurement and Transplantation Network (OPTN) under federal contract.
All board members are volunteers and serve terms ranging from one to three years, depending on the office to which they are elected. Their terms of service begin on July 1, 2018.
Sue Dunn, RN, B.S.N., M.B.A., currently the Vice President/President-Elect, will assume the presidency. She is president and chief executive officer of Donor Alliance in Denver.
Maryl Johnson, M.D., will become the Vice President/President-Elect. She is professor of medicine, heart failure and heart transplantation at the University of Wisconsin Hospitals and Clinics.
Deanna Santana, B.S., will become the Vice President for Patient and Donor Affairs. She is senior public education coordinator at Sierra Donor Services in Sacramento, Calif. She is also a donor mother and a living donor.
Theresa Daly, M.S., RN, B.S.N., FNP, will become the Secretary. She is director of transplant clinical operations at New York Presbyterian/Columbia Medical Center.
Other newly elected members are as follows:
Immediate Past President – Yolanda Becker, M.D., University of Chicago Medicine
Region 3 Councillor – Christopher Anderson, M.D., University of Mississippi Medical Center
Region 4 Councillor – Steven Potter, M.D., FACS, East Texas Medical Center
Region 5 Councillor – Kunam Reddy, M.D., Mayo Clinic, Phoenix
Region 6 Councillor – Susan Orloff, M.D., FACS, AASLD, Oregon Health & Science University
Region 9 Councillor – Rob Kochik, Finger Lakes Donor Recovery Network
At Large Abdominal Surgery Representative –, Rene Romero, M.D., Children’s Healthcare of Atlanta
At Large Hepatology Representative – Simon Horslen, M.B., Ch.B., Seattle Children’s Hospital
At Large Nephrology Representatives:
Eileen Brewer, M.D., Texas Children’s Hospital
Jerry McCauley, M.D., M.P.H., FACP, Thomas Jefferson University Hospital
At Large Pulmonology Representative – Marc Schecter, M.D., Children’s Hospital Medical Center, Cincinnati
At Large Transplant Administrator Representative – Timothy Stevens, RN, B.S.N. CCTC, Sacred Heart Medical Center
OPO Representative – Diane Brockmeier, RN, B.S.N., M.H.A., Mid-America Transplant Services
Histocompatibility Representative – Walter Herczyk, MT, CHS, Gift of Life Michigan Histocompatibility Laboratory
Transplant Coordinator Representative – Mary Francois, RN, M.S., CCTC, NATCO
Medical/Scientific Organization Representatives:
Sharon Bartosh, M.D., University of Wisconsin Hospital and Clinics
Charles Miller, M.D., Cleveland Clinic Foundation
Patient and Donor Affairs Representatives:
Randee Bloom, Ph.D., M.B.A., RN
Rosemary Berkery, J.D.
Laura DePiero, RN, B.S.N.
Joseph Hillenburg
The Organ Procurement and Transplantation Network (OPTN) offers policy proposals for public comment from January 22 through March 23, 2018.
Comments and replies will be published on the OPTN public comment page, to promote transparency and trust in the national transplant system. Visitors can also share comments on social media, if they wish.
Feedback for selected proposals will be sought via a response form. This is part of a trial to study potential enhancements to the public comment process. For the proposals using the response form, the comments related to the proposal will be displayed on the public comment page in the same manner as the blog-style responses to all other proposals.
One of the proposals is a draft of an updated OPTN/UNOS strategic plan. The plan, to be finalized by the OPTN/UNOS Board of Directors after public input, will serve as a roadmap to help prioritize the OPTN’s work through 2021 and provide metrics to assess progress toward key goals.
We encourage patients, transplant candidates and recipients, living donors, donor families and transplant professionals to learn more about the proposals below and provide valuable feedback to help shape U.S. organ transplant policy:
- Aligning VCA program membership requirements with other transplant programs
- Modifications to the distribution of deceased donor lungs
- Clarifying informed consent policies for transmissible disease risk
- Concept paper on expedited organ placement
- Reducing reporting burdens and clarify policies on extra vessels
- Guidance on optimizing VCA recovery from deceased donors
- Changes to waiting time criteria for kidney pancreas candidates
- Modifying the lung Transplant Recipient Form to improve post-transplant lung function data
- Revising OPTN Bylaws Appendix L
- White paper on manipulating waitlist priority
- Guidance for ABO subtyping of Blood Type A and AB organ donors
- Concept paper on improving the OPTN/UNOS committee structure
- Guidance on deceased donor requested information
- OPTN/UNOS strategic plan
- Review board guidance on heart candidates with exceptions for HCM and RCM
During 2017, the number of deceased organ donors in the United States topped 10,000 for the first time, according to preliminary data from United Network for Organ Sharing (UNOS), which serves as the national Organ Procurement and Transplantation Network (OPTN) under federal contract. For the year, organs were recovered from 10,281 donors, representing a 3.1 percent increase over 2016 and an increase of 27 percent since 2007.
A total of 34,768 organ transplants were performed in 2017 using organs from both deceased and living donors, according to preliminary data. This total is a 3.4 percent increase over 2016 and marks the fifth consecutive record-setting year for transplants in the United States. Record number of donor organs were recovered and transplants occurred for each of the four most common organs transplanted – kidney, liver, heart and lung.
“We are grateful that more lives are being saved, year after year, thanks to the boundless generosity of organ donors,” said Yolanda Becker, M.D., president of the OPTN/UNOS Board of Directors. “We remain committed to increasing the number of transplants still further to help the many thousands of people in need of a transplant to sustain them and vastly improve their quality of life.”
Approximately 82 percent (28,587) of the transplants performed in 2017 involved organs from deceased donors. Living donor transplants accounted for the remaining 18 percent (6,181). In the 30-year span from 1988 (the first full year national transplant data were collected) through 2017, a total of 721,742 transplants have been performed nationwide.
While the number of potential organ donors varies among different areas of the country due to differences in population size and medical characteristics, increases were noted in many areas. Of the 58 organ procurement organizations (OPOs) coordinating deceased organ donation nationwide, 35 (60 percent) experienced an increase in donors from 2016 to 2017, including at least one OPO in each of UNOS’ 11 regions.
“Donation and transplantation continues to increase across the country,” said Brian Shepard, Chief Executive Officer of UNOS. “We are working with donation and transplantation professionals nationwide to help identify additional transplant opportunities and enhance the efficiency of the organ acceptance process.”
Broadening of clinical criteria for potential donors accounts for some of the ongoing increase in deceased organ donation and transplantation. In 2017, as compared to 2016, a higher proportion of donors had medical characteristics such as donation after circulatory death as opposed to brain death, drug intoxication as a mechanism of death, age of 50 or older, and/or being identified as having increased risk for blood-borne disease.
“As we increase our understanding of medical criteria that contribute to successful transplantation, donation and transplantation professionals have been able to use organs from a wider set of potential donors,” said David Klassen, M.D., UNOS Chief Medical Officer. “In doing so, we continue to carefully balance the opportunity for transplantation with a commitment to maintaining patient safety.”
United Network for Organ Sharing (UNOS) serves as the national Organ Procurement and Transplantation Network (OPTN) under contract with the Department of Health and Human Services, Health Resources and Services Administration. The OPTN brings together medical professionals, transplant recipients and donor families to develop national organ transplantation policy.
At a meeting by teleconference October 3, 2017, the OPTN/UNOS Board of Directors restored full member privileges for University Hospitals of Cleveland, a transplant center in Cleveland, Ohio.
The Board had placed the hospital on probation in June 2016, after peer review of low transplant volume and early-term recipient deaths at its heart transplant program revealed concerns with the program’s quality management protocols. The hospital has since instituted actions that successfully address the previous concerns.
United Network for Organ Sharing (UNOS) serves as the national Organ Procurement and Transplantation Network (OPTN) under contract with the Department of Health and Human Services, Health Resources and Services Administration, Division of Transplantation. The OPTN brings together medical professionals, transplant recipients and donor families to develop national organ transplantation policy.