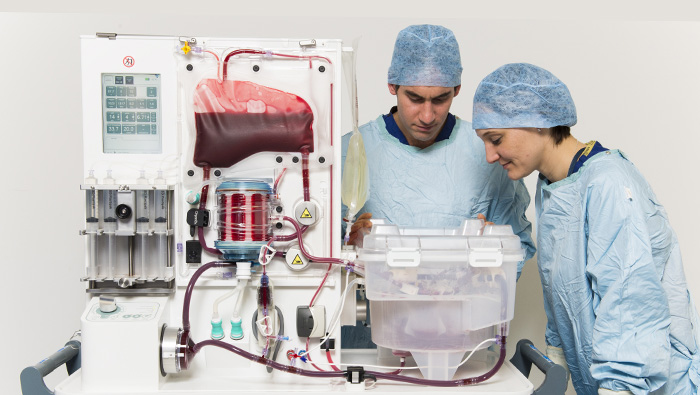
INSIGHTS
Game changers
Companies at the forefront of organ perfusion technology
“We work on the lungs like a team works on a patient.”
Martine Rothblatt, PhD, Chairman & CEO, United Therapeutics
Company headquarters: Silver Spring, Maryland
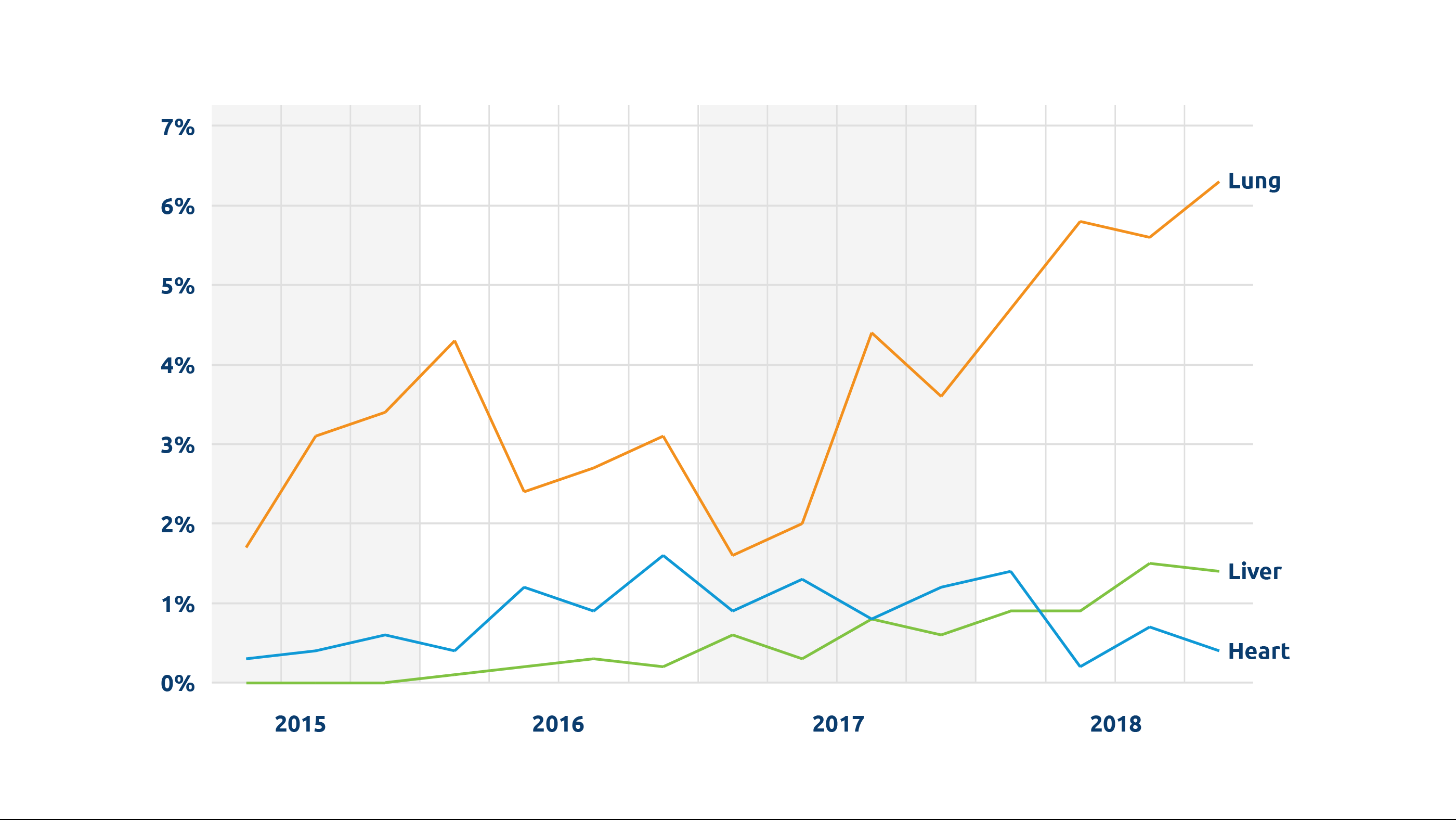
Organ: Lung
Current clinical trials: FDA approval received for Phase 3 trial for 16 centers and 93, or fewer, EVLP transplants.
One significant challenge to widespread implementation of NMP is having the technology and skilled clinical specialists available when needed. Lung Bioengineering, a United Therapeutics subsidiary, seeks to address this challenge with freestanding, centralized perfusion facilities staffed around the clock, to serve multiple transplant centers. Initially unacceptable/marginal lungs are transported to the facility and perfused.
Real-time HD monitoring allows the transplant team to examine the lung remotely during perfusion; if deemed acceptable, the lung is returned to the transplant center. “We work on the lungs like a team works on a patient,” notes United Therapeutics’ Chairman and CEO Martine Rothblatt, PhD. She says during the Phase 2 clinical trial, about two-thirds of the initially unacceptable lungs received by the center were recovered, accepted and successfully transplanted, with perfusion performed on the Toronto EVLP System technology developed at University Health Network in Toronto, Canada. (Lung perfusion services are also available for transplant centers not participating in the trial, with the XVIVO XPS device under the FDA’s HDE approval.)
“Ultimately our goal is to ramp up the availability of lungs for transplant,” says Rothblatt; the company hopes to establish four perfusion centers nationwide with the goal of doubling the number of lung transplants performed annually in the US.

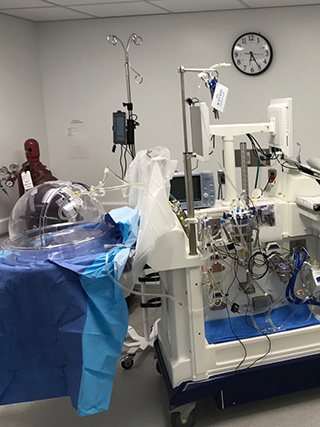
An EVLP Suite

Lung Bioengineering’s headquarters in Silver Spring, Maryland
“Ideally speaking, every organ that is salvageable that has the potential to be transplanted should be assessed and should be allowed time on perfusion technology without impact of ischemia and cold static preservation.”
Tamer Khayal, M.D., Chief Commercial Officer, TransMedics
Company headquarters: Andover, Massachusetts
Organ: Lung, heart, liver
FDA status: PMA for OCS lung, IDE – EA for OCS heart, IDE for OCS liver
Current clinical trials:
- U.S. OCS Heart EXPAND
- U.S. The Organ Care System (OCS
 ) Lung Thoracic Organ Perfusion (TOP) Post Approval Study (PAS) Registry – OCS
) Lung Thoracic Organ Perfusion (TOP) Post Approval Study (PAS) Registry – OCS Lung TOP PAS Registry
Lung TOP PAS Registry - International OCS Lung EXPAND
- International OCS Liver PROTECT
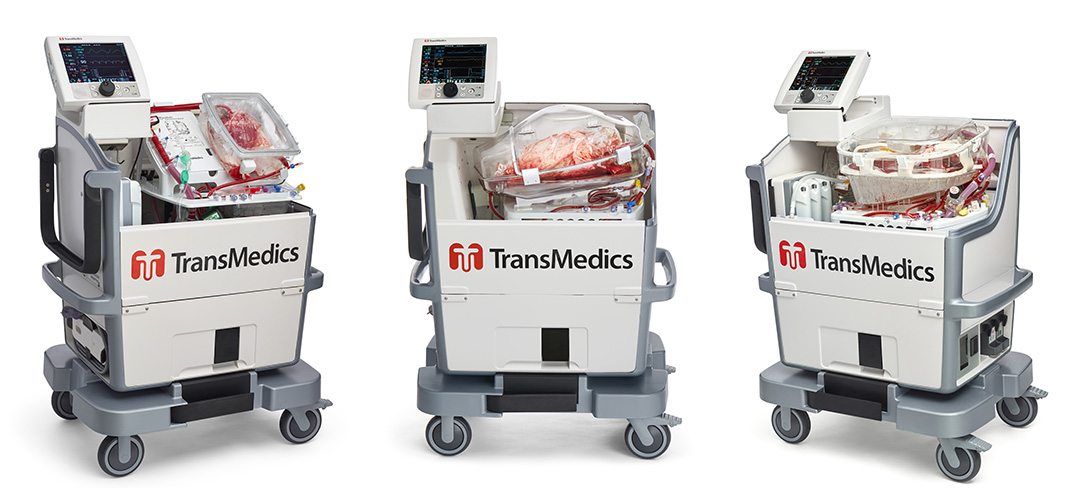
The OCS Lung is currently “the only PMA approved medical technology for ex-vivo perfusion and assessment for routine donor/standard criteria lung transplants in the US,” says TransMedics Chief Commercial Officer Tamer Khayal, M.D. The OCS—which the company has developed for lung, liver, and heart transplantations—is a portable device for NMP that makes it possible to perform perfusion continuously from donor recovery to recipient.
OCS devices were designed to minimize ischemic injury, in comparison to static cold storage preservation. The OCS also allows for diagnostic monitoring of organ viability or function and therapeutic or optimization capabilities during perfusion.
The TransMedics vision is that “ideally speaking, every organ that is salvageable that has the potential to be transplanted should be assessed and should be allowed time on perfusion technology without impact of ischemia and cold static preservation,” says Khayal.
To date, OCS devices have been used in nearly 1,200 transplants worldwide.
“Risk and urgency are the two areas we are tackling head-on with this technology.”
Craig Marshall, CEO, OrganOx
Company headquarters: Oxford, England
Organ: Liver
FDA status: Clinical trial IDE
Current clinical trials: U.S. OrganOx metra®
“Risk and urgency are the two areas we are tackling head-on with this technology,” says OrganOx CEO Craig Marshall.
The metra® (pronounced meetra, from the Greek for womb) is a transportable normothermic machine perfusion (NMP) device that is now in a multi-center Investigational device exemption (IDE) clinical trial in the U.S. comparing the safety and efficacy of preservation and transport on the device to conventional static cold storage (SCS).
“By making it possible to assess the liver ex vivo, we are attempting to reduce the risk associated with lack of information about an organ,” says Marshall. And with clinical data from Europe indicating that livers can be perfused safely for up to 24 hours on the metra®, the device, Marshall says, benefits transplant surgery teams and recipients alike by reducing the urgency of the critical time limits imposed by SCS.
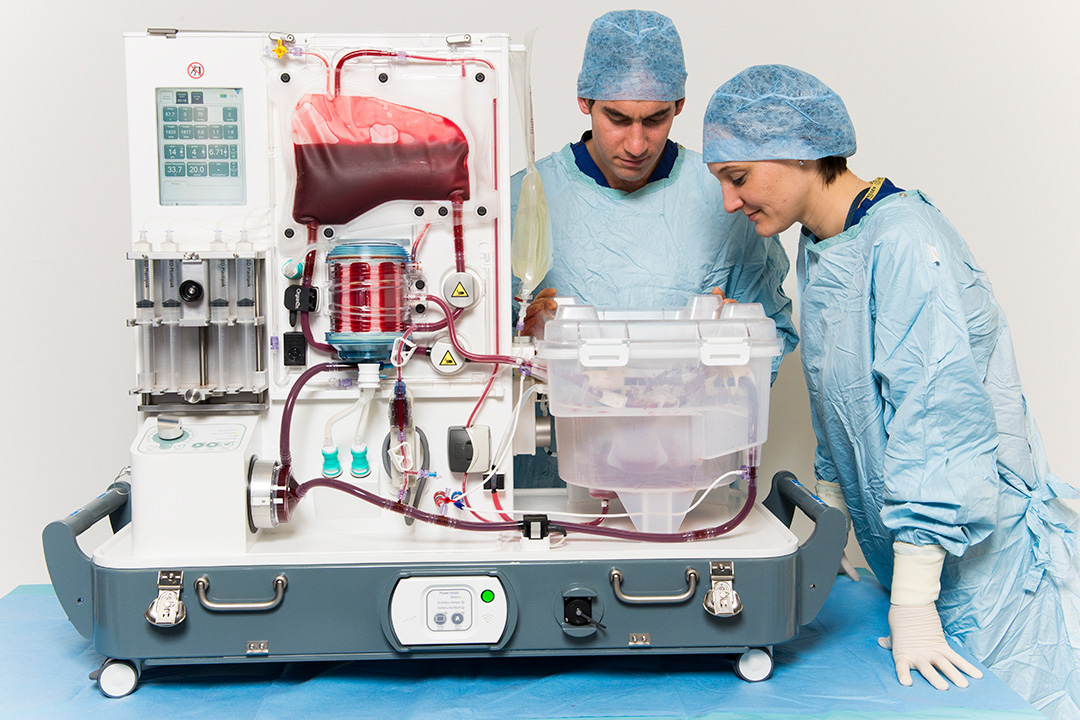
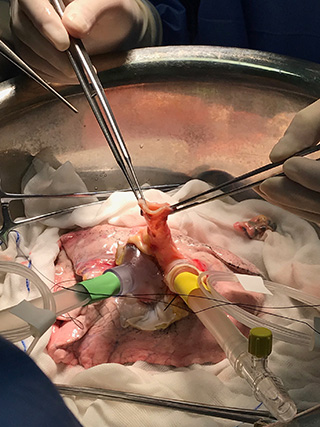
Surgeons David Nasralla and Annemarie Weissenbacher inspecting a liver perfusing on the OrganOx metra® device
“We can offer an additional tool in the clinician’s toolbox to assess lungs that might previously have not been utilized.”
Dan Martinelli, Head of Sales America, XVIVO Perfusion
Company headquarters: Gothenburg, Sweden
Organ: Lung
FDA status: HDE “for flushing and temporary continuous normothermic perfusion of initially unacceptable excised donor lungs.”
Current clinical trials: None
Directed by the company’s vision “that nobody should have to die waiting for an organ,” XVIVO focuses on expanding the use of ex vivo lung perfusion to increase the number of transplantable lungs, says XVIVO Head of Sales Dan Martinelli.
In the completed NOVEL clinical trial, initially unacceptable extended criteria/marginal donor lungs were assessed for transplant via NMP on the XPS device.
Before perfusion, “the majority of those lungs were turned down by the majority of the transplant centers” enrolled in the trial, says Martinelli. Those found acceptable following perfusion and active evaluation, however, were successfully transplanted and demonstrated outcomes—including three-year survival rates—comparable to standard criteria lungs.
“It is the first trial in the U.S. that specifically looked at marginal lungs” and resulted in the FDA’s HDE approval for the XPS, notes XVIVO clinical research program manager Jaya Tiwari. Now, says Martinelli, the XPS can offer “an additional tool in the clinician’s toolbox” to assess lungs that might previously have not been utilized.
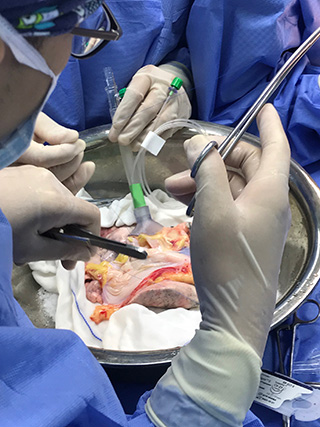
Photos courtesy of XVIVO Perfusion
Learn more
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world.
FDA Terms
The post appeared first on UNOS.