INSIGHTS
UNOS Chief Medical Officer David Klassen, M.D., discusses perfusion-driven advances and remaining challenges
Q. Where is perfusion having the most impact right now in organ transplantation?
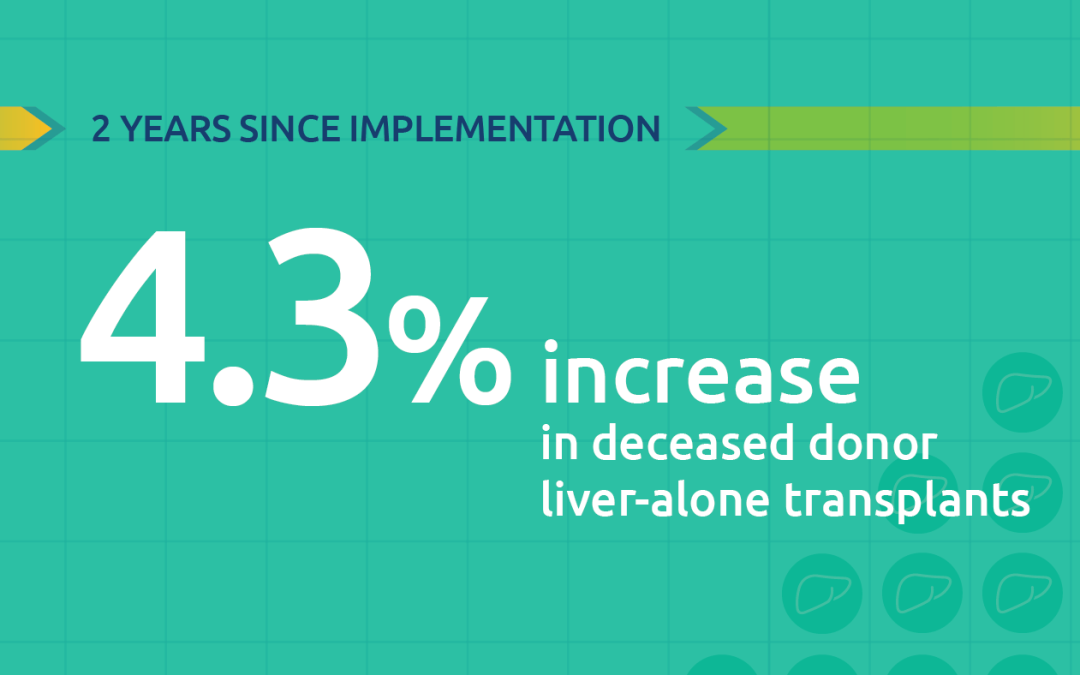
We’re seeing a steady increase in the number of organs being perfused. In 2022, more than 1,100 transplanted donor livers, hearts and lungs were perfused. Between 2018 and 2022, there was an almost five-fold increase in perfused livers transplanted, from fewer than 100 to nearly 500.
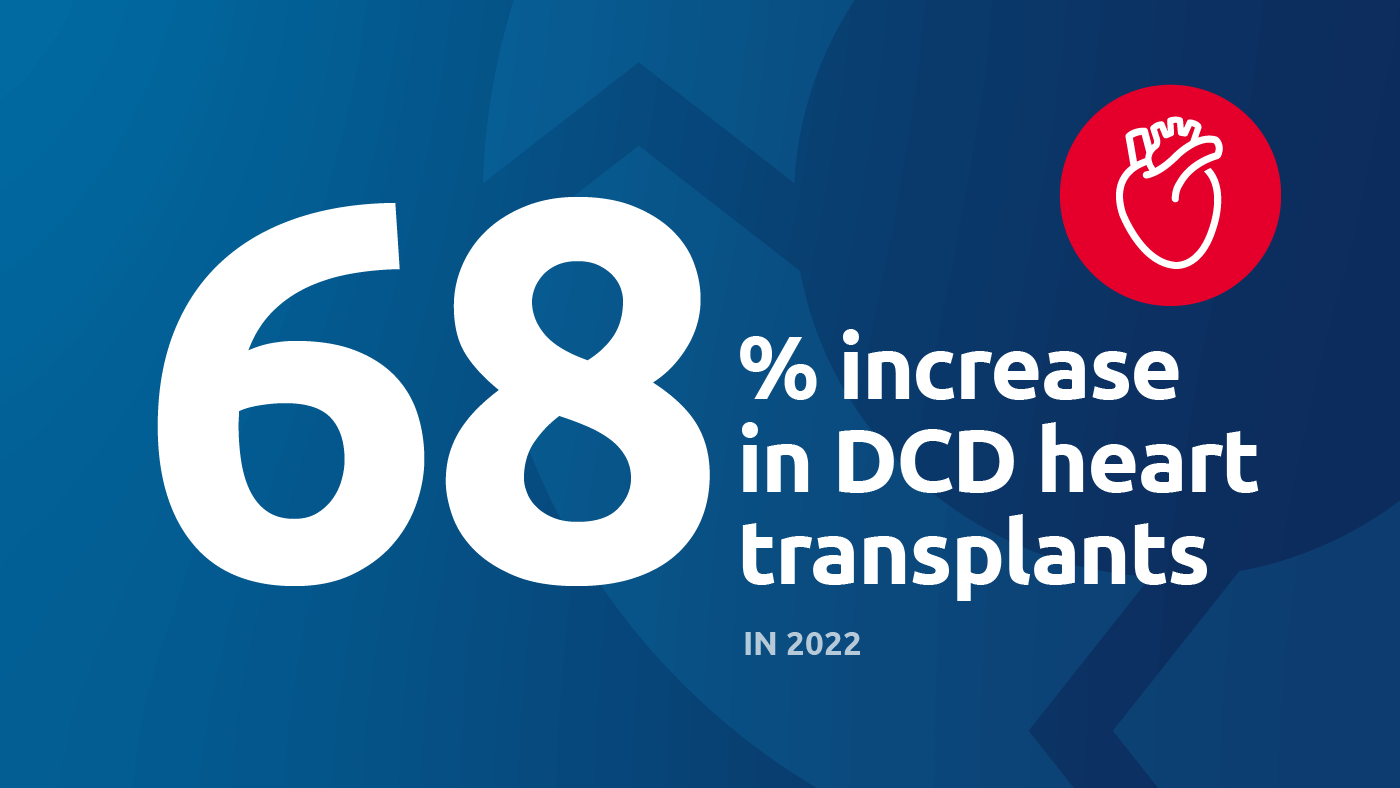
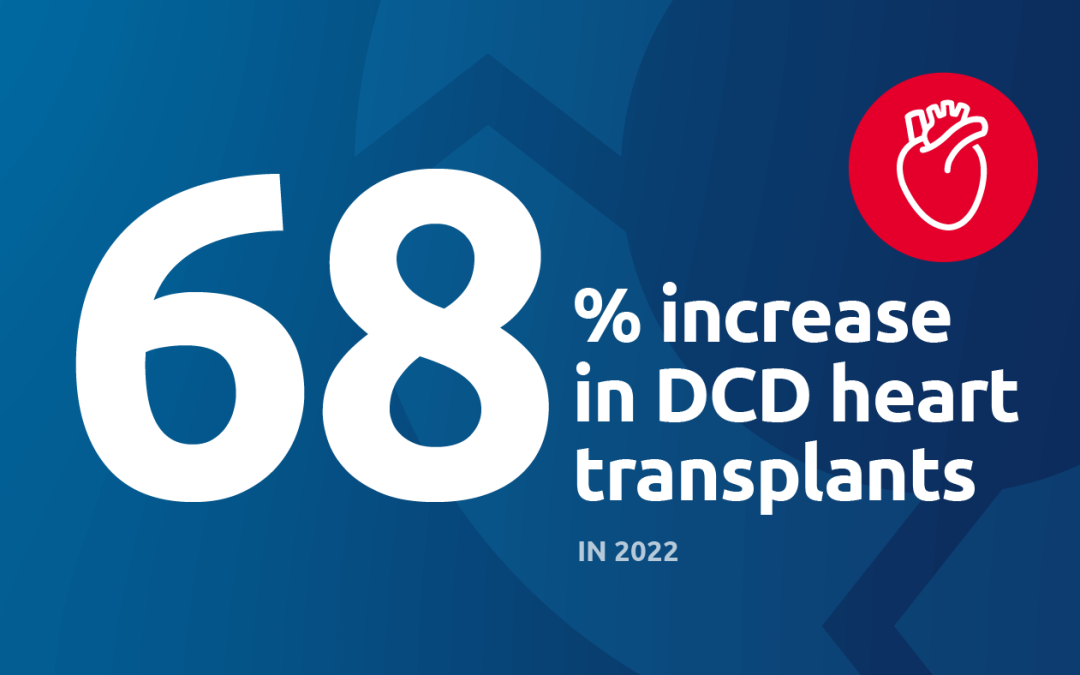
Perhaps the most important recent development is perfusion for DCD (donation after circulatory death) heart transplantation. Because the heart is particularly vulnerable to warm ischemic injury, increasing the number of successful DCD heart transplants was really dependent on perfusion technologies. A third of all donors are DCD donors, and potentially a significant number could be heart donors; one study estimated that widespread adoption of DCD heart transplant could lead to 300 more adult heart transplants annually. UNOS data show that the number of DCD hearts perfused and transplanted has gone from 0 in 2018 to 199 in 2022.

“Perhaps the most important recent development is perfusion for DCD (donation after circulatory death) heart transplantation.”
David Klassen, M.D., Chief Medical Officer
Q. Are there other significant new developments?
In addition to ex-vivo machine perfusion, normothermic regional perfusion (NRP) for recovery of organs from DCD donors is now spreading fairly widely, and is also helping to increase the number of DCD hearts available for transplant. (See Normothermic Regional Perfusion: A reading list.) With NRP, cardiopulmonary bypass or ECMO is used to restore circulation and enable perfusion of DCD organs prior to recovery. Because these technologies are already in use in most ICUs, there is no new technology to acquire or learn to use, as there is with ex-vivo machines. Also with NRP, separate perfusion devices for each organ aren’t needed, as abdominal and thoracic organs can be perfused before recovery with this process.
Q. What barriers remain to greater use of perfusion?
At the present time cost remains the most significant limiting factor for either type of perfusion. It’s expensive, and questions have yet to be resolved about when it is most appropriate to use perfusion, who pays for it, and how those costs are reimbursed. Whether cost will limit the use of perfusion to larger and better-resourced transplant centers or OPOs remains to be seen.
Implementation of these technologies is also logistically complex. It takes time and effort for new technologies and procedures like these to be widely incorporated.
Finally, more data are needed to firmly determine whether perfusion leads to better patient outcomes. Research so far indicates generally equal outcomes when compared with non-perfused organs. Despite these questions perfusion is enabling increased use of expanded criteria and DCD organs, including those that previously might not have been considered viable for transplant.
A reading list
Understanding Normothermic Regional Perfusion (NRP)
Rather than perfusing donor organs by machine after recovery (“ex-vivo” perfusion), NRP uses extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass technology to restore circulation and perfuse DCD donor organs prior to recovery from the deceased donor. The advantages of NRP include the potential to reduce warm ischemia time for DCD donor organs and the ability to assess DCD hearts prior to recovery.
However, NRP is technically complex and requires rapid, coordinated execution by a skilled team. To ensure success with the procedure, the recovery team may need to bring all the necessary equipment and supplies, as well as its own perfusionists, which can add to the cost and other considerations of procurement.
In addition, questions have been raised even within the medical community about the ethics of a procedure that restores circulation in a deceased donor as well as about the transparency necessary for true informed consent from donor families.
This reading list provides an overview of NRP as well as discussions and recent perfusion news coverage.
The Organ Donation and Transplantation Alliance: “Introduction to NRP and Perfusion in DCD: What Do These Concepts Mean?”
American Society of Transplantation Position Statement on Normothermic Regional Perfusion
Nov. 2021 | The Journal of Heart and Lung Transplantation: Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion
June 2022 | Cureus: Normothermic Regional Perfusion is an Emerging Cost-Effective Alternative in Donation After Circulatory Death (DCD) in Heart Transplantation
April 2022 | The Journal of Heart and Lung Transplantation: Hospital Administration Considerations for Implementation of Normothermic Regional Perfusion DCD Heart Transplant Program
March 2022 | The Organ Donation and Transplantation Alliance: “DCD in Heart Transplant – The Case for Normothermic Regional Perfusion” (video)
Oct. 2022 | American Society of Anesthesiologists: “Statement on Controlled Organ Donation After Circulatory Death”
April 2021 | The American College of Physicians: “The American College of Physicians says organ procurement method raises significant ethical concerns”
Feb. 2020 | The Journal of Medicine and Philosophy: A Forum for Bioethics and Philosophy of Medicine: “Why DCD donors are dead” An ethical and philosophical analysis of NRP and DCD transplant.
Nov. 2022 | Mayo Clinic News Release: “Beating the Odds for a transplant”
Sept. 2022 | In tctMD from the Cardiovascular Research Foundation: “Heart Transplantation After Circulatory Death Gives ‘Encouraging’ Results”
Sept. 2022 | UC San Diego Health press release: “Father’s Life is Saved after Receiving Heart, Kidney and Liver Transplant”
May 2022 | KFOR news: “Oklahoma organ team makes history with first successful perfusion liver transplant”
Dec. 2021 | “Medical City Heart Hospital performs first DCD transplant in TX”
Jan. 2020 | PR Newswire: “NYU Langone Performs First U.S. Heart Transplant Using Novel Organ Revitalization Technique”
Dec. 2019 | CNN: “Doctors ‘reanimate’ heart for first-of-its-kind transplant in US”
The post appeared first on UNOS.